Abstract
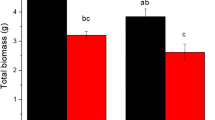
Sources of competition for limited soil resources, such as nitrogen, include competitive interactions among different plant species and between plants and soil microbes. We hypothesized that plant interactions intensified plant competition for inorganic nitrogen with soil microorganisms. To test these competitive interactions, one dominant species (Kobresia humilis Serg) and one less abundant gramineous herb (Elymus nutans Griseb) in an alpine ecosystem were selected as target species to grow under interactions with their neighboring plants and without interaction treatments in field plots. 15N-labeled ammonium and nitrate were used to quantify their partition between plants and soil microorganisms for 48 h after tracer additions. Responses of K. humilis to interactions from their surrounding plants were negative, while those of E. nutans were positive. Species identity, inorganic nitrogen forms, and plant interactions significantly affected the total amount of nitrogen utilization by soil microorganisms and plants. Although plant interactions have negative effects on nitrogen uptake of K. humilis, there is an increase in microbial immobilization of nitrogen under presence of its neighbors. For E. nutans, facilitation from surrounding plants is in favor of their nitrogen uptake. Compared with K. humilis, competition for \( ^{{15}} {\text{N}} - {\text{NO}}^{ - }_{3} \) and \( ^{{15}} {\text{N}} - {\text{NH}}^{ + }_{4} \) was less intensive between E. nutans and microorganisms. \( ^{{15}} {\text{N}} - {\text{NH}}^{ + }_{4} \) recovery by soil microorganisms and plants were not more than or much lower than their utilization of \( ^{{15}} {\text{N}} - {\text{NO}}^{ - }_{3} \) under different interaction treatments. These results suggested that the partitioning of inorganic nitrogen between plants and soil microorganisms is mediated by plant–plant interactions and interactions between plants and soil microorganisms.



Similar content being viewed by others
References
Bokhari UG (1977) Regrowth of western wheatgrass utilizing 14C [carbon isotope]-labeled assimilates stored in belowground parts. Plant Soil 48:115–127
Bremner JM (1965) Inorganic forms of nitrogen. In: Black CA (ed) Methods of soil analysis, vol 2. American Society of Agronomy, Madison, WI, p 1179–1237
Brooker RW (2006) Plant–plant interactions and environmental change. New Phytol 171:271–284
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Buchmann N (2000) Biotic and abiotic factors controlling soil respiration rates in Picea abies stands. Soil Biol Biochem 32:1625–1635
Cahill JF Jr (1999) Fertilization effects on interactions between above- and belowground competition in an old field. Ecology 80:466–480
Cahill JF Jr (2002a) What evidence is necessary in studies which separate root and shoot competition along productivity gradients? J Ecol 90:201–205
Cahill JF Jr (2002b) Interactions between root and shoot competition vary among species. Oikos 99:101–112
Cahill JF Jr (2003) Lack of relationship between below-ground competition and allocation to roots in 10 grassland species. J Ecol 91:532–540
Callaway RM (1998) Are positive interactions species-specific? Oikos 82:202–207
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Michalet R, Paolini L, Pugnaire FI, Newingham B, Aschehoug ET, Armas C, Kikodze D, Cook BJ (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Cao GM, Zhang JX (2001) Soil nutrition and substance cycle of kobresia meadow. In: Zhou XM (ed) Chinese kobresia meadows. Science, Beijing, pp 58–147 (In Chinese)
Casper BB, Jackson RB (1997) Plant competition underground. Ann Rev Ecolog Syst 28:545–570
Cheng XM, Bledsoe CS (2004) Competition for inorganic and organic N by blue oak (Quercus douglasii) seedlings, an annual grass, and soil microorganisms in a pot study. Soil Biol Biochem 36:135–144
Chinese soil taxonomy research group (1995) Chinese soil taxonomy. Beijing, Science, p 58–147
Choler P, Michalet R, Callaway RM (2001) Facilitation and competition on gradients in alpine plant communities. Ecology 82:3295–3308
Clarholm M (1985) Interaction of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil Biol Biochem 17:181–187
Davidson EA, Eckert RW, Hart SC, Firestone MK (1989) Direct extraction of microbial biomass nitrogen from forest and grassland soils of California. Soil Biol Biochem 21:773–777
Farley RA, Fitter AH (1999) Temporal and spatial variation in soil resources in a deciduous woodland. J Ecol 87:688–696
Goldberg DE, Rajaniemi T, Gurevitch J, Stewart-Oaten A (1999). Empirical approaches to quantifying interaction intensity: competition and facilitation along productivity gradients. Ecology 80:1118–1131
Hamilton EW, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82(9):2397–2402
Harte J, Kinzig P (1993) Mutualism and competition between plants and decomposers: implications for nutrient allocation in ecosystems. Am Nat 141:829–846
Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen?. Trends Plant Sci 5:304–308
Jackson LE, Schimel JP, Firestone MK (1989) Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biol Biochem 21:409–415
Jaeger III CH, Monson RK, Fisk MC, Schmidt SK (1999) Seasonal partitioning of nitrogen by plants and soil microorganisms in an alpine ecosystem. Ecology 80(6):1883–1891
Johnson DW (1992) Nitrogen retention in forest soils. J Environ Qual 21:1–12
Jonasson S, Chapin FS Jr, Shaver GR (2001) Biogeochemistry in the Arctic: patterns, processes and controls. In: Schulze ED, Harrison, SP, Heimann, M, Holland EA, Lloyd JJ, Prentice IC, Schimel, D (eds) Global biogeochemical cycles in the climate system. Academic, San Diego, pp 139–150
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV, Callaghan TV (1996) Microbial biomass C, N and P in two arctic soils and responses to addition of NPK fertilizer and sugar: implications for plant nutrient uptake. Oecologia 106:507–515
Kalembasa SJ, Jenkinson DSA (1973) Comparative study of titrimetric and gravimetric methods for determination of organic carbon in soil. J Sci Food Agric 24:1085–1090
Kaye JP, Hart SC (1997) Competition for nitrogen between plants and soil microorganisms. Trends Ecol Evol 12:139–143
Killham K (1994) Soil ecology. Cambridge University Press, Cambridge, MA
Kronzucker HJ, Siddiqi MY, Glass ADM (1997) Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385:59–61
Malhi SS, Nyborg M (1988) Control of nitrification of fertilizer nitrogen: effect of inhibitors, bending and nesting. Plant Soil 107(2):245–250
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, San Diego, CA
Norton JM, Firestone MK (1996) N dynamics in the rhizosphere of Pinus ponderosa seedlings. Soil Biol Biochem 28:351–362
Nye PH, Tinker PB (1977) Solute movement in the soil–root system[M]. Blackwell Scientific, Oxford
Pearson J, Stewart GR (1993) The deposition of atmospheric ammonia and its effects on plants. New Phytol 125:283–305
Pruden G, Powlson DS, Jenkinson DS (1985) The measurement of 15N in soil and plant material. Fertil Res 6:205–218
Qiu B, Du GZ (2004) Light competition can cause a decline in diversity with increased productivity in an alpine meadow. Acta Bot Boreal Occident Sin 24(9):1646–1650
Reynolds HL, Packer A, Bever JD, Clay K (2003) Grass roots ecology: plant–microbe–soil interactions as drivers of plant community structure and dynamics. Ecology 84(9):2281–2291
Schimel JP, Jackson LE, Firestone MK (1989) Spatial and temporal effects on plant-microbial competition for inorganic nitrogen in a California annual grassland. Soil Biol Biochem 21:1059–1066
Schlesinger WH (1991) Biogeochemistry: an analysis of global change. Academic, San Diego, CA
Schweitzer JA, Bailey JK, Rehill BJ, Martinsen GD, Hart SC, Lindroth RL, Keim P, Whitham TG (2004) Genetically based trait in a dominant tree affects ecosystem processes. Ecol Lett 7:127–134
Scott NA, Binkley D (1997) Litter quality and annual net N mineralization: comparisons across sites and species. Oecologia 111:151–159
Song MH, Tian YQ, Xu XL, Hu QW, Ouyang H (2006) Interactions between root and shoot competition among four plant species in an alpine meadow on the Tibetan Plateau. Acta Oecol 29:214–220
Stark JM, Hart SC (1997) High rates of nitrification and nitrate turnover in undisturbed coniferous forests. Nature 385:61–64
Twolan-Strutt L, Keddy PA (1996) Above- and belowground competition intensity in two contrasting wetland plant communities. Ecology 77:259–270
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115
Whipps JM (1990) Carbon economy. In: Lynch JM, Wiley J (eds) The rhizosphere. Wiley, New York, pp 52–97
Wilson SD, Tilman D (1991) Components of plant competition along an experimental gradient of nitrogen availability. Ecology 72:1050–1065
Wilson SD, Tilman D (1995) Competitive responses of eight old-field plant species in four environments. Ecology 76:1169–1180
WRB (1998) World reference base for soil resources. FAO/ISRIC/ISSS, Rome
Xu XL, Ouyang H, Pei ZY, Zhou CP (2003) Fate of 15N labeled nitrate and ammonium salts added to an alpine meadow in the Qinghai-Xizang Plateau, China. Acta Bot Sinica 45(3):276–281
Zak DR (1990) The vernal dam: plant–microbe competition for nitrogen in northern hardwood forest. Ecology 71:651–656
Zheng D (2000) Mountain geoecology and sustainable development of the Tibetan Plateau. Kluwer, Dordrecht, The Netherlands
Zhou XM (2001) Chinese kobresia meadows. Science, Beijing (In Chinese)
Acknowledgements
We appreciate Dr. Martin Werth, from Institute of Soil Science and Land Evaluation, University of Hohenheim, Germany, for his meticulous work in improving the language usage of our manuscript. This research was funded by the National Natural Science Foundation for Young Scientists of China (30600070), and the National Basic Research program of China (Grant no.2005CB422005), and the Key Project of the Chinese Academy of Sciences (KZCX3-SW-339-04). Our experiments were performed in accordance with the current laws of our country.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Herbert Johannes Kronzucker.
Rights and permissions
About this article
Cite this article
Song, M., Xu, X., Hu, Q. et al. Interactions of plant species mediated plant competition for inorganic nitrogen with soil microorganisms in an alpine meadow. Plant Soil 297, 127–137 (2007). https://doi.org/10.1007/s11104-007-9326-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9326-1