Abstract
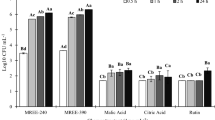
The effect of cellulase and pectinase on bacterial colonization of wheat was studied by three different experiments. In the first experiment, the root colonization of 3 wheat cultivars (Ghods, Roshan and Omid) by two A. brasilense strains (Sp7 and Dol) was compared using pre-treated roots with cellulase and pectinase, and non-treated with these enzymes (control). Although the root colonization varied greatly among strain-plant combinations in controls, the pre-treatment of roots with polysaccharide degrading enzymes significantly increased the bacterial count in roots, regardless of the strain-plant combination. This might be an indication that cell wall may act as an important factor in plant-Azospirillum interaction. In the second experiment, the root cellulase activity of the same wheat cultivars treated with and without the two Azospirillum brasilense, strains (Sp7 and Dol) was compared. The pre-treatment of wheat roots with Azospirillum enhanced the cellulase activity of wheat root extracts. Thus, the cellulase activity might participate in the initial colonization of wheat roots by Azospirillum. The comparison of the cellulase activity of root extracts within inoculated and non-inoculated seedlings showed that the inoculation had enhanced the cellulase activity in root extracts, but this effect was directly dependent on the strain-plant combination. Strain Sp7 stimulated the highest cellulase activity in cv. Roshan, but strain Dol induced the highest enzyme activity in cv. Ghods. In the third experiment, several growth parameters of those 3 wheat cultivars treated with and without those two bacterial strains (Sp7 and Dol) were compared. The highest magnitude of growth responses caused by Sp7 strain was in the cv Roshan, but Dol strain stimulated the highest growth in cv Ghods. Therefore, effective colonization may contribute to more growth responses.



Similar content being viewed by others
References
Al-Mallah MK, Davey MR, Cocking EC (1987) Enzymatic treatment of clover root hairs removes a barrier to Rhizobium-host specificity. Nat Biotechnol 5:1319–1322
Al-Mallah MK, Davey MR, Cocking EC (1989) Formation of nodular structures on rice seedlings by rhizobia. J Exp Bot 40:473–478
Amooaghaie R (2001) Investigation of interaction between A. brasilense and root cells of wheat in initial stages of root inoculation. PhD Thesis, University of Isfahan, Isfahan, Iran
Amooaghaie R, Mosatjeran A, Emtiazi G (2002b) The effect of compatible and incompatible Azospirillum brasilense strains on proton efflux of intact wheat roots. Plant Soil 243:155–160
Amooaghaie R, Mosatjeran A, Emtiazi G (2002a) The effect of strain and concentration of Azospirillum brasilense bacterium on growth and development of root in wheat cultivars. Iranian J Agricultural Sci 33:213–222
Assmus B, Hutzler P, Kirchhof G, Amann R, lawrence JR, Hartmann A (1995) In situ localization of Azospirillum brasilense in the rhizosphere of wheat with fluorescently labelled, rRNA-targeted oligonucleotide probes and scanning confocal laser microscopy. Appl Environ Microbiol 61:1013–1019
Baldani VLD, Baldani JI, Doebereiner J (1983) Effects of Azospirillum inoculation on root infection and nitrogen incorporation in wheat. Can J Microbiol 29:924–929
Bashan Y (1986) Enhancement of wheat root colonization and plant development by Azospirillum brasilense Cd. following temporary depression of the rhizosphere microflora. Appl Environ Microbiol 51:1067–1071
Bashan Y, Holguin G (1993) Anchoring of Azospirillum brasilense to hydrophobic polystyrene and wheat roots. J Gen Microbiol 139:379–385
Bashan Y, Holguin G (1997) Azospirillum plant relationships: environmental and physiological advances (1990–1996). Can J Microbiol 43:103–121
Bashan Y, Levanony H (1989) Factors affecting adsorption of Azospirillum brasilense Cd to root hairs as compared with root surface of wheat. Can J Microbiol 35:936–944
Bashan Y, Levanony H (1985) An improved selection technique and medium for the isolation and enumeration of Azospirillum brasilense. Can J Microbiol 31:947–952
Bhattarai T, Hess D (1993) Yield responses of Nepalese spring wheat (T. aestivum) cultivars to inoculation with Azospirillum spp. of Nepalese origin. Plant Soil 151:67–76
Day JM, Dobereiner J (1976) Physiological aspects of N2-fixation by a spirillum from digitaria roots. Soil Biol Biochem 12:433–439
Dazzo FB, Napoli CA, Hubbell DH (1976) Adsorption of bacteria to roots as related to host specificity in the Rhizobium–clover symbiosis. Appl Environ Microbiol 32:166–171
Del Gallo M, Negi M, Neyra CA (1989) Calcofluor and lectin-binding exocellular polysaccharides of A. brasilense and A. lipoferum. J Bacteriol 171:3504–3510
Gafny R, Okon Y, Kapulnik Y, Fischer M (1986) Adsorption of Azospirillum brasilense to corn roots. Soil Biol Biochem 18:69–75
Garcia de Salomone I, Dobereiner J (1996) Maize genotype effects on the response to Azospirillum inoculation. Biol Fertil Soil 21:193–196
Hoagland DR, Arnon DL (1950) The water-culture method of growing plants without soil. Calif Agric Exp Stn 374:1–32
Hubbell DH (1981) Legume infection by Rhizobium: a conceptual approach. BioScience 31:832–837
Jain DK, Patriquin G (1984) Root hair deformation, bacterial attachment, and plant growth in wheat-Azospirillum associations. Appl Environ Microbiol 48:1208–1213
Kapulnik Y, Okon Y, Heins Y (1985) Changes in root morphology of wheat caused by Azospirillum inoculation. Can J Microbiol 31:881–887
Khammas KM, Kaiser P (1991) Characterization of a pectinolytic activity in Azospirillum irakense. Plant Soil 137:75–79
Levanony H, Bashan Y, Romono B, Klein E (1989) Ultrastructural localization and identification of A. brasilense Cd on and within wheat root by immono-gold labelling. Plant Soil 117:207–218
Mandel M, Weber J (1969) Exoglucanase activities of microorganisms. Adv Chem 95:391–414
Mateos PF, Jimenez-Zurdo JI, Chen L, Squartini AS, Haack SK, Martinez-Molina E, Hubbell DH, Dazzo FB (1992) Cell-associated pectinolytic and cellulolytic enzymes in Rhizobium leguminosarum biovar trifolii. Appl Environ Microbiol 58:1816–1822
Morales VM, Monila EM, Hubbell DH (1984) Cellulase production by Rhizobium. Plant Soil 80:407–415
Mostajeran A, Amooaghaie R, Emtiazi G (2002) Root hair density and deformation of inoculated roots of wheat cultivars by A. brasilense and role of IAA in this phenomenon. Iranian Biol J 13:18–28
Myers ML, Hubbell DH (1987) Plant cell wall carbohydrates as substrates for Azospirillum brasilense. Appl Environ Microbiol 53:2745–2748
Okon Y (1985) Azospirillum as a potential inoculant for Agriculture. Trends Biotechnol 3:223–228
Okon Y, Kapulnik Y (1986) Development and function of Azospirillum-inoculated roots. Plant Soil 90:3–16
Patriquin DG, Döbereiner J, Jain DX (1983) Sites and processes of association between diazotrophs and grasses. Can J Microbiol 29:900–915
Plazinski J, Rolfe BG (1985) Analysis of the pectolytic activity of Rhizobium and Azospirillum strains isolated from Trifolium repens. J Plant Physiol 120:181–187
Puente ME, Bashan Y (1993) Effect of inoculation with Azospirillum brasilense strains on the germination and seedling growth of the giant columnar cardon cactus (Parchycereus pringlei). Symbiosis 15:49–60
Rao PSK, Arnuachalam V, Tilak K (1990) Genotype dependent response to Azospirillum treatment in yield and nitrogenase activity in Brassica juncea. Curr Sci 59:605–609
Reinhold-Hurek B, Hureck T, Claeyssens M, Van-Montasu M (1993) Cloning expression in Escherchia Coli and Characterization of cellulolytic enzyrnes of Azoarcus sp. J Bacteriol 175:7056–7065
Reinhold B, Hureck T (1988) Location of diazotrophs in the root interior with special attention to the kallar grass association. Plant Soil 110:259 268
Rothballer M, Schmid M, Hartmann A (2003) In situ localization and PGPR-effect of Azospirillum brasilense strains colonizing roots of different wheat varieties. Symbiosis 34:261–279
Schloter M, Hartmann A (1998) Endophytic and surface colonization of wheat roots (Triticum aestivum) by different Azospirillum brasilense strains studied with strain-specific monoclonal antibodies. Symbiosis 25:159–179
Tien TM, Diem HG, Gaskins MH, Hubbell DH (1981) Polygalacturonic acid transeliminase production by Azospirillum species. Can J Microbiol 27:426–431
Umali-Garcia M, Hubbell DH, Gashins MH, Dazzo FB (1980) Association of Azospirillum with grass roots. Appl Environ Microbiol 39:219–226
Acknowledgment
This work was supported by research grant of University of Esfahan in Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mostajeran, A., Amooaghaie, R. & Emtiazi, G. The participation of the cell wall hydrolytic enzymes in the initial colonization of Azospirillum brasilense on wheat roots. Plant Soil 291, 239–248 (2007). https://doi.org/10.1007/s11104-006-9189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9189-x