Abstract
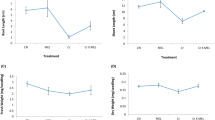
Growth, lipid peroxidation, H2O2 produciton and the response of the antioxidant enzymes and metabolites of the ascorbate glutathione pathway to oxidative stress caused by two concentrations (50 and 100 µM) of Cr(III) and Cr(VI) was studied in 15 day old seedlings of sorghum (Sorghum bicolor (L.) Moench cv CO 27) after 10 days of treatment. Cr accumulation in sorghum plants was concentration and organ dependant. There was no significant growth retardation of plants under 50 µM Cr(III) stress. 100 µM Cr(VI) was most toxic of all the treatments in terms of root and leaf growth and oxidative stress. 50 µM Cr(VI) treated roots exhibited high significant increase in superoxide dismutase (SOD), dehydroascorbate reductase (DHAR) and glutathione reductase (GR) (p < 0.01) and significant increases in catalse (CAT), ascorbate peroxidase (APX) and monodehydroascorbate reductase (MDHAR) (p < 0.05). A high increase in ascorbic acid (AA) level was seen in roots of 50 µM Cr(VI) treated plants in comparison with control. Levels of reduced glutathione (GSH) showed a varied and complex response in all the treatments in both plant parts. GSH/GSSG ratio was not affected by Cr(III) treatment in leaves, in contrast, roots exhibited significant reduction in the ratio. Results indicate that GSH depletion increased sensitivity to oxidative stress (Cr(VI) roots and leaves and Cr(III) 100 µM roots) and AA in tandem with APX compensated for GSH depletion by acting directly on H2O2 and the mechanism of defensive response in roots as well as leaves varied in its degree and effectiveness due to the concentration dependant differences observed in translocation of the element itself, reactive oxygen species (ROS) generation and enzyme inhibition based on the oxidation state supplied to the plants.
Similar content being viewed by others
References
Alscher R G 1989 Biosynthesis and antioxidant function of glutathione in plants. Physiol. Plantarum 77: 457–64.
Behra T H, Panda S K and Patra H K 1999 Chromium ion induced lipid peroxidation in developing wheat seedlings: Role of growth hormones. Indian J. Plant Physiol. 4: 236–238.
Bergmeyer H U, Gawehn K and Grassi M 1974 Enzymes as biochemical reagents. In: Methods in Enzymatic analysis. pp 425–522. Academic Press, New York, USA.
Beyer W F and Fridovich I 1987 Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 161: 559–566.
Bradford M M 1976 A rapid and sensitive method for quantification of microgram quantities utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Brauer S L and Wetterhahn K E 1991 Chromiumn (VI) forms thiolate complex with glutathione. J. Am. Chem. Soc. 113: 3001–3007.
Brauer S L Hneihen A S McBride J S Wetterhahn K E 1996 Chromium forms thiolate complexes with λ-glutamylcysteine, N-acetylcysteine, cysteine and the methyl ester of N-acetylcysteine. Inorg. Chem. 35: 373–381.
Chen Z, Young T E, Ling J, Chang S C and Gallie D R 2003 Increaseing vitamin C content of plants through snhanced ascorbate recycling. Proceedings of the Natioanal Academy of Science USA 100, 3525–3530.
Clijsters H, Cuypers A and Vangronsveld J 1999 Physiological responses to heavy metals in plants; Defence against oxidative stress. Zeitschr. Naturforsch 54c: 730–734.
Davies F T, Puryear J D, Newton R J, Egilla J N and Grossi J A S 2002 Mycorrhizal fungi increase chromium uptake by sunflower plants: Influence on tissue mineral concentration, growth, and gas exchange. J. Plant Nutr. 25: 2389–2407.
Desmet G A, de Ruyter and Rigoet A 1975 Absorption and metabolism of Cr(VI) by isolated chloroplasts. Phytochemisty 14: 2585–2588.
Dixit V, Pandey V and Shyam R 2002 Chromium ions inactivate electron transport and enhance superoxide generation in vivo in pea (Pisum sativum L.cv. Azad) root mitochondria. Plant Cell Environ. 25: 687–690.
Foyer C H, Dujardyn M and Lemoine Y 1989 Responses of photosynthesis and xanthophylls and ascorbate glutathione cycle to changes in irradiance, photo inhibition and recovery. Plant Physiol. Biochem. (Paris) 27: 751–760.
Gerbling K P, Graham J K, Fischer K H and Latzco E 1984 Partial purification and properties of soluble ascorbate peroxidases from pea leaves. J. Plant Physiol. 115: 59–67.
Grill E, Löffler S, Winnacker E L and Zenk M H 1989. Phytochelatins, the heavy-metal binding peptides of plants, are synthesized from glutathione by a specific λ glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 286: 6838–6842.
Gupta M, Cuypers A, Vangronsveld J and Clijsters H 1999 Copper effects the enzymes of the ascorbate glutathione cycle and its related metabolites in the roots of Phaseolus vulgaris. Physiol. Plant. 106: 262–267.
Hodges D M, Andrews C J, Johnson D A and Hamilton R I 1996 Antioxidant compound responses to chilling stress in different sensitive inbred maize lines. Physiol. Plant. 98: 685–692.
Hussain N A and Asada K 1984 Monodehydroacorbate reductase from spinach chloroplasts and its characterisation as a thiol enzyme. Plant Cell Physiol. 25: 85–92.
Jimenez A, Hernandez J A, del Rio L A and Sevilla F 1997 Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 114: 275–284.
Jung S 2003 Expression level of specific isozymes of maize catalase mutants influences other antioxidants on norflurazon-induced oxidative stress. Pesticide Biochem. Physiol. 75: 9–17.
Kotas J and Stasicka Z 2000 Commentary: Chromium occurrence in the environment and methods of its speciation. Environ. Poll. 107: 263–283.
May M J, Vernoux T, Leaver C, Montagu M V and Inze D 1998 Glutathione homeostasis in plants: implications for environmental sensing and plant development. J. Exp. Bot. 49: 649–667.
McGrath S P 1982 The uptake and translocation of tri- and hexavalent chromium and effects on the growth of oat in flowing nutrient solution and in soil. New Phytol. 92: 381–390.
Mei B, Puryear J D and Newton R J 2002 Assessment of Cr tolerance and accumulation in selected plant species. Plant Soil 247: 223–231.
Micera G and Dessi A 1988 Chromium adsorption by plant roots and formation of long lived Cr(V) species: and ecological hazard? J. Inorg. Biochem. 43: 157–166.
Mittler R 2002. Oxidative stress, antioxidants and stress tolerance, Trends Plant Sci. 7: 405–410.
Morell S, Follmann H, De Tullio M and Haberlein I 1997. Dehydroascorbate and dehydroascorbate reductase are phantom indicators of oxidative stress in plants. FEBS Lett. 414: 567–570.
Noctor G, Arisi A-CM, Jouanin L, Kunert K-J, Rennenberg H and Foyer C H 1998 Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 49: 623–647.
Noctor G and Foyer C H 1998 Ascorbate and glutathione: keeping active oxygen under control. Ann. Rev. Plant Physiol. Plant Mol. Biol. 49: 249–279.
Okuda T, Matsuda Y, Yamanaka A and Sagisaka S 1991 Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 97: 1265–1267.
Paciolla C, De Tullio M C, Chiappetta A, Innocenti A M, Bitonti M B, Liso R and Arrigoni O 2001 Short- and Long-Term Effects of Dehydroascorbate in Lupinus albus and Allium cepa Roots. Plant Cell Physiol. 42: 857–863.
Pekker I, Elisha T O and Mittler R 2002 Reactive oxygen intermediates and glutathione regulate the expression of cytosolic ascorbate peroxidase during iron-mediated oxidative stress in bean. Plant Mol. Biol. 49: 429–438.
Potters G, Gara L D, Asard H and Horemans N 2002 Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol. Biochem. 40: 537–548.
Rock M L, James B and Helz G R 2001 Hydrogen peroxide effects on Cr oxidation state and solubility in four diverse, Cr-enriched soils. Environmental Sci. Technol. 35: 4054–4059.
Samantary S 2002 Biochemical responses of Cr-tolerant and Cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere 47: 1065–1072.
Shewry P R and Peterson P J 1974 The uptake of chromium by barley seedlings (Hordeum vulgare L.) J. Exp. Bot. 25: 785–797.
Skeffington R A, Shewry P R and Petersen P J 1976 Chromium uptake and transport in barley seedlings Hordeum vulgare, Planta 132: 209–214.
Smith I K, Vierhellaer T L and Thorne C A 1988 Assay of glutathione reductase in crude tissue homogenates using 5.5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 175: 408–413.
Stohs S J and Bagachi D 1995 Oxidative mechanism in the toxicity of metal ions. Free Radicals Biol. Med. 18: 321–336.
Suzuki Y and Fukuda K 1990 Reduction of hexavalent chromium by ascorbic acid and glutathione with special reference to the rat lung. Arch Toxicol 64: 169–176.
Toppi L S D, Fossati F, Musetti R, Mikerezi I and Favali M A 2002 Effects of hexavalent chromium on maize, tomato, and cauliflower plants. J. Plant Nutr. 25: 701–717.
Von Burg R and Liu D 1993 Chromium and hexavalent chromium. J. Appl. Toxicol. 13: 225–230.
Wallace A, Soufi S M, Cha J W and Romney E M 1976 Some effects of chromium toxicity on bush bean plants grown in soil. Plant Soil 44: 471–473.
Wilkinson L, Hill M, Welna J P and Birkenbevel B K 1996 Systat for windows. Version 6 edition. SPSS Inc., Evanston, IL, USA.
Willekens H, Inzé D, Van Montagu M and Van Camp W 1995 Catalase in plants. Molecular Breeding 1: 207–228.
Zayed A, Lytle C M, Qian-JinHong, Terry N and Qian J H 1998 Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 206: 293–299.
Zayed A and Terry N 2003 Chromium in the environment: factors affecting biological remediation. Plant Soil 249: 139–156.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shanker, A.K., Pathmanabhan, G. Speciation dependant antioxidative response in roots and leaves of sorghum (Sorghum bicolor (L.) Moench cv CO 27) under Cr(III) and Cr(VI) stress. Plant Soil 265, 141–151 (2004). https://doi.org/10.1007/s11104-005-0332-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-005-0332-x