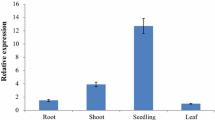
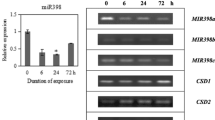
Abstract
Cadmium (Cd) is a non-essential heavy metal, assimilated in plant tissue with other nutrients, disturbing the ions’ homeostasis in plants. The plant develops different mechanisms to tolerate the hazardous environmental effects of Cd. Recently studies found different miRNAs that are involved in Cd stress. In the current study, miR397 mutant lines were constructed to explore the molecular mechanisms of miR397 underlying Cd tolerance. Compared with the genetically modified line of overexpressed miR397 (artificial miR397, amiR397), the lines of downregulated miR397 (Short Tandem Target Mimic miR397, STTM miR397) showed more substantial Cd tolerance with higher chlorophyll a & b, carotenoid and lignin content. ICP-OES revealed higher cell wall Cd and low total Cd levels in STTM miR397 than in the wild-type and amiR397 plants.
Further, the STTM plants produced fewer reactive oxygen species (ROS) and lower activity of antioxidants enzymes (e.g., catalase [CAT], malondialdehyde [MDA]) compared with amiR397 and wild-type plants after stress, indicating that silencing the expression of miR397 can reduce oxidative damage. In addition, the different family transporters’ gene expression was much higher in the amiR397 plants than in the wild type and STTM miRNA397. Our results suggest that miR397 plays a role in Cd tolerance in Arabidopsis thaliana. Overexpression of miR397 could decrease Cd tolerance in plants by regulating the expression of LAC 2/4/17, changing the lignin content, which may play an important role in inducing different stress-tolerant mechanisms and protecting the cell from a hazardous condition. This study provides a basis to elucidate the functions of miR397 and the Cd stress tolerance mechanism in Arabidopsis thaliana.
Key message
The miR397 modified lines influence the lignin and Cd content in the plants. The amiR397 plants susceptible to Cd stress have less lignin and high Cd content than STTM miR397 plants, changing the underlying stress regulatory pathways.










Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Abdel-Ghany SE, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem 283:15932–15945
Arcuri MLC, Fialho LC, Nunes-Laitz AV, Fuchs-Ferraz MCP, Wolf IR, Valente GT, Marino CL, Maia IG (2020) Genome-wide identification of multifunctional laccase gene family in Eucalyptus grandis: potential targets for lignin engineering and stress tolerance. Trees 34:745–758
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Bai Y, Ali S, Liu S, Zhou J, Tang Y (2022) Characterization of plant laccase genes and their functions. Gene 852:147060
Bandeoglu E, Eyidogan F, Yucel M, Oktem HA (2004) Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul 42:69–77
Bao W, Omalley DM, Whetten R, Sederoff RR (1993) A laccase associated with lignification in loblolly-pine xylem. Science 260:672–674
Basso MC, Cerrella EG, Cukierman AL (2004) Cadmium uptake by lignocellulosic materials: Effect of lignin content. Sep Sci Technol 39:1163–1175
Berthet S, Demont-Caulet N, Pollet B, Bidzinski P, Cézard L, Le Bris P, Borrega N, Hervé J, Blondet E, Balzergue S et al (2011) Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell 23:1124–1137
Blaschek L, Murozuka E, Ménard D, Pesquet E (2022) Different combinations of laccase paralogs non-redundantly control the lignin amount and composition of specific cell types and cell wall layers in Arabidopsis. BioRxiv 53:1
Budak H, Kantar M, Bulut R, Akpinar BA (2015) Stress responsive miRNAs and isomiRs in cereals. Plant Sci 235:1–13
Chen H, Zhang C, Guo H, Hu Y, He Y, Jiang D (2018a) Overexpression of a Miscanthus sacchariflorus yellow stripe-like transporter MsYSL1 enhances resistance of Arabidopsis to cadmium by mediating metal ion reallocation. Plant Growth Regul 85:101–111
Chen X, Ouyang Y, Fan Y, Qiu B, Zhang G, Zeng F (2018b) The pathway of transmembrane cadmium influx via calcium-permeable channels and its spatial characteristics along rice root. J Exp Bot 69:5279–5291
Chen X, Tao H, Wu Y, Xu X (2022) Effects of Cadmium on metabolism of photosynthetic pigment and photosynthetic system in Lactuca sativa L revealed by physiological and proteomics analysis. Sci. Hortic. 305:111371
Chi M, Bhagwat B, Tang G, Xiang Y (2016) Knockdown of polyphenol oxidase gene expression in potato (Solanum tuberosum L) with artificial microRNAs. In: Fett-Neto AG (ed) Biotechnology of plant secondary metabolism: methods and protocols. Springer, pp 163–178
Dahuja A, Kumar RR, Sakhare A, Watts A, Singh B, Goswami S, Sachdev A, Praveen S (2021) Role of ATP-binding cassette transporters in maintaining plant homeostasis under abiotic and biotic stresses. Physiol Plant 171(4):785–801
De Boer AH, Volkov V (2003) Logistics of water and salt transport through the plant: structure and functioning of the xylem. Plant Cell Environ 26(1):87–101
Di Toppi LS, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41(2):105–130
Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V (2021) The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front Plant Sci 11:552969
Dwivedi UN, Singh P, Pandey VP, Kumar A (2011) Structure–function relationship among bacterial, fungal and plant laccases. J Mol Catal B Enzym 68:117–128
Fang X, Zhao Y, Ma Q, Huang Y, Wang P, Zhang J, Nian H, Yang C (2013) Identification and comparative analysis of cadmium tolerance-associated miRNAs and their targets in two soybean genotypes. PLoS ONE 8(12):e81471
Feng J, Jia W, Lv S, Bao H, Miao F, Zhang X, Wang J, Li J, Li D, Zhu C, Li S, Li Y (2018) Comparative transcriptome combined with morpho-physiological analyses revealed key factors for differential cadmium accumulation in two contrasting sweet sorghum genotypes. Plant Biotechnol J 16(2):558–571. https://doi.org/10.1111/pbi.12795
Feng YZ, Yu Y, Zhou YF, Yang YW, Lei MQ, Lian JP, He H, Zhang YC, Huang W, Chen YQ (2020) A natural variant of miR397 mediates a feedback loop in circadian rhythm. Plant Physiol 182(1):204–214
Gaillard S, Jacquet H, Vavasseur A, Leonhardt N, Forestier C (2008) AtMRP6/AtABCC6, an ATP-binding cassette transporter gene expressed during early steps of seedling development and upregulated by cadmium in Arabidopsis thaliana. BMC Plant Biol 8:22
Garcia JS, Dalmolin ÂC, Cortez PA, Barbeira PS, Mangabeira PA, França MG (2018) Short-term cadmium exposure induces gas exchanges, morphological and ultrastructural disturbances in mangrove Avicennia schaueriana young plants. Mar Pollut Bull 131:122–129
Ge Y, Song Q, Li ZA (2015) Mannich base biosorbent derived from alkaline lignin for lead removal from aqueous solution. J Ind Eng Chem 23:228–234
Gravot A, Lieutaud A, Verret F, Auroy P, Vavasseur A, Richaud P (2004) AtHMA3, a Plant P1B-ATPase, functions as a Cd/Pb transporter in yeast. FEBS Lett 561:22–28
Guleria P, Mahajan M, Bhardwaj J, Yadav SK (2011) Plant small RNAs: biogenesis, mode of action and their roles in abiotic stresses. Genom Proteom Bioinform 9(6):183–199
Guo Z, Yuan X, Li L, Zeng M, Yang J, Tang H, Duan C (2022) Genome-wide analysis of the ATP-binding cassette (ABC) transporter family in Zea mays L and its response to heavy metal stresses. Int J Mol Sci 23(4):2109
Gupta OP, Meena NL, Sharma I, Sharma P (2014) Differential regulation of microRNAs in response to osmotic, salt and cold stresses in wheat. Mol Biol Rep 41:4623–4629
Hong J, Wang C, Wagner DC, Gardea-Torresdey JL, He F, Rico CM (2021) Foliar application of nanoparticles: mechanisms of absorption, transfer, and multiple impacts. Environ Sci Nano 8(5):1196–1210
Hu H, Brown PH (1994) Localization of boron in cell walls of squash and tobacco and its association with pectin (evidence for a structural role of boron in the cell wall). Plant Physiol 105(2):681–689
Huang S, Zhou J, Gao L, Tang Y (2021) Plant miR397 and its functions. Funct Plant Biol 48(4):361–370
Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14(6):787–799
Khandal H, Singh AP, Chattopadhyay D (2020) The MicroRNA397b-LACCASE2 module regulates root lignification under water and phosphate deficiency. Plant Physiol 182(3):1387–1403
Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y (2007) The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J 50:207–218
Li LZ, Tu C, Wu LH, Peijnenburg WJ, Ebbs S, Luo YM (2017a) Pathways of root uptake and membrane transport of Cd (2+) in the zinc/cadmium hyperaccumulating plant sedum plumbizincicola. Environ Toxicol Chem 36:1038–1046
Li R, Kang C, Song X, Yu L, Liu D, He S, Zhai H, Liu Q (2017b) A ζ-carotene desaturase gene, IbZDS, increases β-carotene and lutein contents and enhances salt tolerance in transgenic sweetpotato. Plant Sci 262:39–51
Li Q, Feng J, Chen L, Xu Z, Zhu Y, Wang Y, Xiao Y, Chen J, Zhou Y, Tan H, Zhang L (2019) Genome-wide identification and characterization of Salvia miltiorrhiza laccases reveal potential targets for salvianolic acid B biosynthesis. Front Plant Sci 10:435
Liu Y, Liu S, Deng Y, You C, Zhang W, Zhou J, Chen X, Gao L, Tang Y (2020) Genome-wide mRNA and small RNA transcriptome profiles uncover cultivar-and tissue-specific changes induced by cadmium in Brassica parachinensis. Environ Exp Bot 180:104207
Liu S, Li L, Deng Y, Bai Y, Sun C, Huang S, Zhou J, Shi L, Yang X, Li L, Chen X (2022) BrpNAC895 and BrpABI449 coregulate the transcription of the afflux-type cadmium transporter BrpHMA2 in Brassica parachinensis. Hortic. Res. 9:uhac044
Lu S, Li Q, Wei H, Chang MJ, Tunlaya-Anukit S, Kim H, Liu J, Song J, Sun YH, Yuan L, Yeh TF (2013) Ptr-miR397a is a negative regulator of laccase genes affecting lignin content in Populus trichocarpa. Proc Natl Acad Sci 110(26):10848–10853
Lux A, Martinka M, Vaculík M, White PJ (2011) Root responses to cadmium in the rhizosphere: a review. J Exp Bot 62(1):21–37
Mani A, Sankaranarayanan K (2022) Natural resistance-associated macrophage proteins (NRAMPs): functional significance of metal transport in plants. Plant metal and metalloid transporters. Singapore, Springer Nature Singapore, pp 91–107
Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, Katou K, Kodama I, Sakurai K, Takahashi H, Satoh-Nagasawa N (2011) OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189(1):190–199
Morel M, Crouzet J, Gravot A, Auroy P, Leonhardt N, Vavasseur A, Richaud P (2009) AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol 149:894–904
Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y (2012) The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J 69:278–288
Pérez Chaca MV, Vigliocco A, Reinoso H, Molina A, Abdala G, Zirulnik F, Pedranzani H (2014) Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.) Merr. Acta Physiol Plant 36:2815–2826
Qamer Z, Chaudhary MT, Du X, Hinze L, Azhar MT (2021) Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L in response to extreme abiotic conditions. J Cotton Res 4(1):1–9
Ranocha P, Chabannes M, Chamayou S, Danoun S, Jauneau A, Boudet AM, Goffner D (2002) Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol 129:145–155
Rizvi A, Zaidi A, Ameen F, Ahmed B, AlKahtani MD, Khan MS (2020) Heavy metal induced stress on wheat: phytotoxicity and microbiological management. RSC Adv 10(63):38379–38403
Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, Del Rio LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52(364):2115–2126
Sasaki A, Yamaji N, Ma JF (2014) Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J Exp Bot 65(20):6013–6021. https://doi.org/10.1093/jxb/eru340
Seregin IV, Kozhevnikova AD (2011) Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ J Plant Physiol 55:1–22
Shahid M, Dumat C, Khalid S, Niazi NK, Antunes PM (2017) Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol 241:73–137
Singh VP, Srivastava PK, Prasad SM (2012) Differential effect of UV-B radiation on growth, oxidative stress and ascorbate-glutathione cycle in two cyanobacteria under copper toxicity. Plant Physiol Biochem 61:61–70
Song XQ, Liu LF, Jiang YJ, Zhang BC, Gao YP, Liu XL, Lin QS, Ling HQ, Zhou YH (2013) Disruption of secondary wall cellulose biosynthesis alters cadmium translocation and tolerance in rice plants. Mol Plant 6:768–780
Song Y, Jin L, Wang X (2017) Cadmium absorption and transportation pathways in plants. Int J Phytoremediat 19:133–141
Sunkar R, Zhu JK (2004) Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell 16(8):2001–2019
Sunkar R, Li YF, Jagadeeswaran G (2012) Functions of microRNAs in plant stress responses. Trends Plant Sci 17(4):196–203
Swetha C, Basu D, Pachamuthu K, Tirumalai V, Nair A, Prasad M, Shivaprasad PV (2018) Major domestication-related phenotypes in Indica rice are due to loss of miRNA-mediated laccase silencing. Plant Cell 30:2649–2662
Taïbi K, Taïbi F, AitAbderrahim L, Ennajah A, Belkhodja M, Mulet JM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312
Tang G, Yan J, Gu Y, Qiao M, Fan R, Mao Y, Tang X (2012) Construction of short tandem target mimic (STTM) to block the functions of plant and animal microRNAs. Methods 58(2):118–125
Telfer A (2014) Singlet oxygen production by PSII Under light stress: mechanism, detection and the protective role of beta-carotene. Plant Cell Physiol 55:1216–1223
Trchounian A, Petrosyan M, Sahakyan N (2016) Plant cell redox homeostasis and reactive oxygen species. Redox state as a central regulator of plant-cell stress responses. Springer, Cham, pp 25–50
Tripathy BC, Oelmüller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7(12):1621–1633
Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Meth 3(1):1–12
Vatehova-Vivodova Z, Kollarova K, Malovikova A, Liskova D (2018) Maize shoot cell walls under cadmium stress. Environ Sci Pollut Res 25:22318–22322
Verret F, Gravot A, Auroy P, Leonhardt N, David P, Nussaume L, Vavasseur A, Richaud P (2004) Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and Plant metal tolerance. FEBS Lett 576:306–312
Wang CY, Zhang S, Yu Y, Luo YC, Liu Q, Ju C, Zhang YC, Qu LH, Lucas WJ, Wang X, Chen YQ (2014) MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotechnol J 12(8):1132–1142
Wang B, Wen JL, Sun SL, Wang HM, Wang SF, Liu QY, Charlton A, Sun RC (2017) Chemosynthesis and structural characterization of a novel lignin-based bio-sorbent and its strong adsorption for Pb (II). Ind Crops Prod 108:72–80
Wang P, Yang B, Wan H, Fang X, Yang C (2018) The differences of cell wall in roots between two contrasting soybean cultivars exposed to cadmium at young seedlings. Environ Sci Pollut Res 25:29705–29714
Wang X, Gao W, Zhao P, Yu C, Liu H, Nie Z, Qin S, Li C (2019) Changes to wheat seedling root morphology in response to cadmium stress. J Agro-Environ Sci 38(6):1218–1225
Wojas S, Hennig J, Plaza S, Geisler M, Siemianowski O, Sklodowska A, Ruszczynska A, Bulska E, Antosiewicz DM (2009) Ectopic expression of Arabidopsis ABC transporter MRP7 modifies cadmium root-to-shoot transport and accumulation. Environ Pollut 157:2781–2789
Wong CKE, Cobbett CS (2009) HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol 181(1):71–78
Xu L, Wang Y, Zhai L, Xu Y, Wang L, Zhu X, Gong Y, Yu R, Limera C, Liu L (2013) Genome-wide identification and characterization of cadmium-responsive microRNAs and their target genes in radish (Raphanus sativus L.) roots. J Exp Bot 64(14):4271–4287
Yang Y-J, Cheng L-M, Liu Z-H (2007) Rapid effect of cadmium on lignin biosynthesis in soybean roots. Plant Sci 172:632–639
Yao N, Greenberg JT (2006) Arabidopsis accelerated cell death2 modulates programmed cell death. Plant Cell 18(2):397–411
Yokoyama R, Nishitani K (2006) Identification and characterization of Arabidopsis thaliana genes involved in xylem secondary cell walls. J Plant Res 119:189–194
Zhang Y, Culhaoglu T, Pollet B, Melin C, Denoue D, Barriere Y, Baumberger S, Méchin V (2011) Impact of lignin structure and cell wall reticulation on maize cell wall degradability. J Agricult Food Chem 59(18):10129–10135
Zhang YC, Yu Y, Wang CY, Li ZY, Liu Q, Xu J, Liao JY, Wang XJ, Qu LH, Chen F, Xin P (2013) Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nat Biotechnol 31(9):848
Zhao W, Li Z, Fan J, Hu C, Yang R, Qi X, Chen H, Zhao F, Wang S (2015) Identification of jasmonic acid- associated microRNAs and characterization of the regulatory roles of the miR319/TCP4 module under root-knot nematode stress in tomato. J Exp Bot 66(15):4653–4667
Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L (2010) Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J Exp Bot 61(15):4157–4168. https://doi.org/10.1093/jxb/erq237
Zhou ZS, Song JB, Yang ZM (2012) Genome-wide identification of Brassica napus microRNAs and their targets in response to cadmium. J Exp Bot 63(12):4597–4613
Funding
This work was supported by Shenzhen Natural Science Fund (the Stable Support Plan Program, 20220804115333001), Natural Science Foundation of Guangdong province (2020A1515010309), Chinese National Key R & D Project for Synthetic Biology (2018YFA0902500), National Natural Science Foundation of China (32273118), Guangdong Key R & D Project (2022B1111070005), Shenzhen Special Fund for Sustainable Development (KCXFZ20211020164013021) and Shenzhen University 2035 Initiative to Dr. Zhangli Hu.
Author information
Authors and Affiliations
Contributions
SA, SH, and YT designed, analyzed, and prepared the manuscript. SA, SH, and JZ assisted with experiments. YB, YL, LS, SL, and ZH evaluated data and revised the manuscript. All authors contributed to the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, S., Huang, S., Zhou, J. et al. miR397-LACs mediated cadmium stress tolerance in Arabidopsis thaliana. Plant Mol Biol 113, 415–430 (2023). https://doi.org/10.1007/s11103-023-01369-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-023-01369-x