Abstract
Key message
Rice leucine-rich repeat extensin-like protein OsPEX1 mediates the intersection of lignin deposition and plant growth.
Abstract
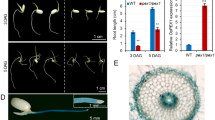
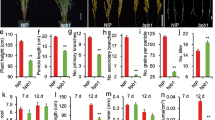
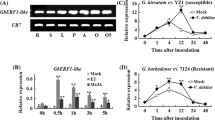
Lignin, a major structural component of secondary cell wall, is essential for normal plant growth and development. However, the molecular and genetic regulation of lignin biosynthesis is not fully understood in rice. Here we report the identification and characterization of a rice semi-dominant dwarf mutant (pex1) with stiff culm. Molecular and genetic analyses revealed that the pex1 phenotype was caused by ectopic expression of a leucine-rich repeat extension-like gene, OsPEX1. Interestingly, the pex1 mutant showed significantly higher lignin content and increased expression levels of lignin-related genes compared with wild type plants. Conversely, OsPEX1-suppresssed transgenics displayed low lignin content and reduced transcriptional abundance of genes associated with lignin biosynthesis, indicating that the OsPEX1 mediates lignin biosynthesis and/or deposition in rice. When OsPEX1 was ectopically expressed in rice cultivars with tall stature that lacks the allele of semi-dwarf 1, well-known green revolution gene, the resulting transgenic plants displayed reduced height and enhanced lodging resistance. Our study uncovers a causative effect between the expression of OsPEX1 and lignin deposition. Lastly, we demonstrated that modulating OsPEX1 expression could provide a tool for improving rice lodging resistance.





Similar content being viewed by others
References
Baumberger N, Ringli C, Keller B (2001) The chimeric leucine-rich repeat/extensin cell wall protein LRX1 is required for root hair morphogenesis in Arabidopsis thaliana. Genes Dev 15:1128–1139
Baumberger N, Doesseger B, Guyot R, Diet A, Parsons RL, Clark MA, Simmons MP, Bedinger P, Goff SA, Ringli C, Keller B (2003a) Whole-Genome comparison of leucine-rich repeat extensins in Arabidopsis and Rice. A conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol 131:1313–1326
Baumberger N, Steiner M, Ryser U, Keller B, Ringli C (2003b) Synergistic interaction of the two paralogous Arabidopsis genes LRX1 and LRX2 in cell wall formation during root hair development. Plant J 35:71–81
Bedinger P (2018) Coordinating cell walls and cell growth: A role for LRX extensin chimeras. Plant Physiol 176:1890
Borassi C, Sede AR, Mecchia MA, Salgado Salter JD, Marzol E, Muschietti JP, Estevez JM (2016) An update on cell surface proteins containing extensin-motifs. J Exp Bot 67:477–487
De Micco V, Aronne G (2007) Combined histochemistry and autofluorescence for identifying lignin distribution in cell walls. Biotech Histochem 82:209–216
Demura T, Ye Z (2010) Regulation of plant biomass production. Curr Opin Plant Biol 13:298–303
Draeger C, Ndinyanka Fabrice T, Gineau E, Mouille G, Kuhn BM, Moller I, Abdou M, Frey B, Pauly M, Bacic A, Ringli C (2015) Arabidopsis leucine-rich repeat extensin (LRX) proteins modify cell wall composition and influence plant growth. BMC Plant Biol 15:1–11
Dunser K, Gupta S, Ringli C, Kleine-Vehn J (2017) LRX- and FER-dependent extracellular sensing coordinates vacuolar size for cytosol homeostasis. bioRxiv. https://doi.org/10.1101/231043
Fabrice TN, Vogler H, Draeger C, Munglani G, Gupta S, Herger AG, Knox P, Grossniklaus U, Ringli C, Ciereszko I (2018) LRX proteins play a crucial role in pollen grain and pollen tube cell wall development. Plant Physiol 176:1981–1992
Ge Z, Bergonci T, Zhao Y, Zou Y, Du S, Liu M, Luo X, Ruan H, García-Valencia LE, Zhong S, Hou S, Huang Q, Lai L, Moura DS, Gu H, Dong J, Wu H, Dresselhaus T, Xiao J, Cheung AY, Qu L (2017) Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science 358:1596–1600
Hake S, Vollbrecht E, Freeling M (1989) Cloning knotted, the dominant morphological mutant in maize using Ds2 as a transposon tag. EMBO J 8:15–22
Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343:408–411
Hirano K, Aya K, Kondo M, Okuno A, Morinaka Y, Matsuoka M (2012) OsCAD2 is the major CAD gene responsible for monolignol biosynthesis in rice culm. Plant Cell Rep 31:91–101
Hirano K, Aya K, Morinaka Y, Nagamatsu S, Sato Y, Antonio BA, Namiki N, Nagamura Y, Matsuoka M (2013) Survey of genes involved in rice secondary cell wall formation through a co-expression network. Plant Cell Physiol 54:1803–1821
Huang D, Wang S, Zhang B, Shang-Guan K, Shi Y, Zhang D, Liu X, Wu K, Xu Z, Fu X, Zhou Y (2015) A Gibberellin-mediated della-nac signaling cascade regulates cellulose synthesis in rice. Plant Cell 27:1681–1696
Kohorn B, Kohorn S (2012) The cell wall-associated kinases, WAKs, as pectin receptors. Front Plant Sci 3:88. https://doi.org/10.3389/fpls.2012.00088
Lisch D (2013) How important are transposons for plant evolution? Nat Rev Genet 14:49–61
Liu Y, Whittier R (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25:674–681
Liu F, Zhang X, Zhang Z, Chen Z, Zhu H, Wang J, Zhang J, Zhang G (2007) Transpositional behaviour of the Ds element in the Ac/Ds system in rice. Chin Sci Bull 52:2789–2796
Liu X, Wolfe R, Welch LR, Domozych DS, Popper ZA, Showalter AM (2016) Bioinformatic identification and analysis of extensins in the plant kingdom. PLoS ONE 11:e150177
Marzol E, Borassi C, Bringas M, Sede A, Rodríguez Garcia DR, Capece L, Estevez JM (2018) Filling the gaps to solve the extensin puzzle. Mol Plant 11:645–658
Mecchia MA, Santos-Fernandez G, Duss NN, Somoza SC, Boisson-Dernier A, Gagliardini V, Martínez-Bernardini A, Fabrice TN, Ringli C, Muschietti JP, Grossniklaus U (2017) RALF4/19 peptides interact with LRX proteins to control pollen tube growth in Arabidopsis. Science 358:1600–1603
Moreira-Vilar FC, Siqueira-Soares RDC, Finger-Teixeira A, Oliveira DMD, Ferro AP, Da Rocha GJ, Ferrarese MDLL, Dos Santos WD, Ferrarese-Filho O (2014) The acetyl bromide method is faster, simpler and presents best recovery of lignin in different herbaceous tissues than klason and thioglycolic acid methods. PLOS ONE 9:e110000
Muehlbauer GJ, Fowler JE, Girard L, Tyers R, Harper L, Freeling M (1999) Ectopic expression of the maize homeobox gene liguleless3 alters cell fates in the leaf. Plant physiol 119:651–662
Nissen KS, Willats WGT, Malinovsky FG (2016) Understanding CrRLK1L function: cell walls and growth control. Trends Plant Sci 21:516–527
Park SH, Kim CM, Je BI, Park SH, Park SJ, Piao HL, Xuan Y, Choe MS, Satoh K, Kikuchi S, Lee KH, Cha YS, Ahn BO, Ji HS, Yun DW, Lee MC, Suh S, Eun MY, Han C (2007) A Ds-insertion mutant of OSH6 (Oryza sativa Homeobox 6) exhibits outgrowth of vestigial leaf-like structures, bracts, in rice. Planta 227:1–12
Peng D, Chen X, Yin Y, Lu K, Yang W, Tang Y, Wang Z (2014) Lodging resistance of winter wheat (Triticum aestivum L.): lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crop Res 157:1–7
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786
Rogers LA, Campbell MM (2004) The genetic control of lignin deposition during plant growth and development. New Phytol 164:17–30
Schneeberger RG, Becraft PW, Hake S, Freeling M (1995) Ectopic expression of the knox homeo box gene rough sheath1 alters cell fate in the maize leaf. Genes Dev 9:2292–2304
Sede AR, Borassi C, Wengier DL, Mecchia MA, Estevez JM, Muschietti JP (2018) Arabidopsis pollen extensins LRX are required for cell wall integrity during pollen tube growth. FEBS Lett 592:233–243
Vollbrecht E, Veit B, Sinha N, Hake S (1991) The developmental gene KNOTTED-1 is a member of a maize homeobox gene family. Nature 350:241–243
Wang X, Wang K, Liu X, Liu M, Cao N, Duan Y, Yin G, Gao H, Wang W, Ge W, Wang J, Li R, Guo Y (2018) Pollen-expressed leucin-rich-repeat extensins are essential for pollen germination and growth. Plant Physiol 176:1993–2006
Yano K, Ookawa T, Aya K, Ochiai Y, Hirasawa T, Ebitani T, Takarada T, Yano M, Yamamoto T, Fukuoka S, Wu J, Ando T, Ordonio RL, Hirano K, Matsuoka M (2015) Isolation of a novel lodging resistance QTL gene involved in strigolactone signaling and its pyramiding with a QTL gene involved in another mechanism. Mol Plant 8:303–314
Yoon J, Choi H, An G (2015) Roles of lignin biosynthesis and regulatory genes in plant development. J Integr Plant Biol 57:902–912
Zhang J, Li G, Song Y, Liu Z, Yang C, Tang S, Zheng C, Wang S, Ding Y (2014) Lodging resistance characteristics of high-yielding rice populations. Field Crops Res 161:64–74
Zhang X, Zheng X, Ke S, Zhu H, Liu F, Zhang Z, Peng X, Guo L, Zeng R, Hou P, Liu Z, Wu S, Song M, Yang J, Zhang G (2016) ER-localized adenine nucleotide transporter ER-ANT1: an integrator of energy and stress signaling in rice. Plant Mol Biol 92:701–715
Zhao C, Zayed O, Yu Z, Jiang W, Zhu P, Hsu C, Zhang L, Tao WA, Lozano-Durán R, Zhu J (2018) Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc Natl Acad Sci USA 115:13123
Zhong R, Ye Z (2007) Regulation of cell wall biosynthesis. Curr Opin Plant Biol 10:564–572
Zhong R, Lee C, McCarthy RL, Reeves CK, Jones EG, Ye Z (2011) Transcriptional activation of secondary wall biosynthesis by rice and maize nac and myb transcription factors. Plant Cell Physiol 52:1856–1871
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 30900884, 31622041, 31471788 and 31671594); by Natural Science Foundation of Guangdong Province, China (Grant Nos. 2014A030313457 and 2015A020209118); by the Hatch Project of National Institute of Food and Agriculture, U.S.D.A (Grant No. 02413).
Author information
Authors and Affiliations
Contributions
SK and XL performed experiments and conducted fieldwork. JL and YHH worked on the transgenic lines. TFH and XQZ designed the experiments and analyzed the data; TFH supervised and complemented the writing; XQZ conceived the project and wrote the article with contributions of all the authors.
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ke, S., Luan, X., Liang, J. et al. Rice OsPEX1, an extensin-like protein, affects lignin biosynthesis and plant growth. Plant Mol Biol 100, 151–161 (2019). https://doi.org/10.1007/s11103-019-00849-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-019-00849-3