Abstract
Key message
Lower promoter activity is closely associated with lower MdPIN1b expression in the M9 interstem, which might contribute to the dwarfing effect in apple trees.
Abstract
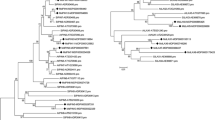
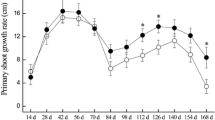
Apple trees grafted onto dwarfing rootstock Malling 9 (M9) produce dwarfing tree architecture with high yield and widely applying in production. Previously, we have reported that in Malus ‘Red Fuji’ (RF) trees growing on M9 interstem and Baleng Crab (BC) rootstock, IAA content was relatively higher in bark tissue of M9 interstem than that in scion or rootstock. As IAA polar transportation largely depends on the PIN-FORMED (PIN) auxin efflux carrier. Herein, we identify two putative auxin efflux carrier genes in Malus genus, MdPIN1a and MdPIN1b, which were closely related to the AtPIN1. We found that MdPIN1b was expressed preferentially in BC and M9, and the expression of MdPIN1b was significantly lower in the phloem of M9 interstem than that in the scion and rootstock. The distinct expression of MdPIN1b and IAA content were concentrated in the cambium and adjacent xylem or phloem, and MdPIN1b protein was localized on cell plasma membrane in onion epidermal cells transiently expressing 35S:MdPIN1b-GFP fusion protein. Interestingly, an MdPIN1b mutant allele in the promoter region upstream of M9 exhibited decreased MdPIN1b expression compared to BC. MdPIN1b over-expressing interstem in tobacco exhibited increased polar auxin transport. It is proposed that natural allelic differences decreased promoter activity is closely associated with lower MdPIN1b expression in the M9 interstem, which might limit the basipetal transport of auxin, and in turn might contribute to the dwarfing effect. Taken together, these results reveal allelic variation underlying an important apple rootstock trait, and specifically a novel molecular genetic mechanism underlying dwarfing mechanism.






Similar content being viewed by others
References
Allen JRF, Baker DA (1980) Free-tryptophan and indole-3-acetic-acid levels in the leaves and vascular pathways of Ricinus communis L. Planta 148:69–74
Atkinson CJ, Else MA, Taylor L, Dover CJ (2003) Root and stem hydraulic conductivity as determinants of growth potential in grafted trees of apple (Malus pumila Mill.). J Exp Bot 54:1221–1229
Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433:39–44
Carraro N, Forestan C, Canova S, Traas J, Varotto S (2006) ZmPIN1a and ZmPIN1b encode two novel putative candidates for polar auxin transport and plant architecture determination of maize. Plant Physiol 142:254–264
Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inze d, Sandberg G, Casero PJ, Bennett M (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13:843–852
Chen D, Ren Y, Deng Y, Zhao J (2010) Auxin polar transport is essential for the development of zygote and embryo in Nicotiana tabacum L. and correlated with ABP1 and PM H+-ATPase activities. J Exp Bot 61:1853–1867
Colla G, Rouphael Y, Cardarelli M, Massa D, Salerno A, Rea E (2006) Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J Hortic Sci Biotechnol 81:146–152
Drews GN, Bowman JL, Meyerowitz EM (1991) Negative regulation of the arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65:991–1002
Feraru E, Friml J (2008) PIN polar targeting. Plant Physiol 147:1553–1559
Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426:147–153
Fukuda H (2004) Signals that control plant vascular cell differentiation. Nat Rev Mol Cell Biol 5:379–391
Galweiler L, Guan CH, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282:2226–2230
Ganguly A, Lee SH, Cho M, Lee OR, Yoo H, Cho HT (2010) Differential auxin-transporting activities of PIN-FORMED proteins in Arabidopsis root hair cells. Plant Physiol 153:1046–1061
Habets MEJ, Offringa R (2014) PIN-driven polar auxin transport in plant developmental plasticity: a key target for environmental and endogenous signals. New Phytol 203:362–377
Immanen J, Nieminen K, Smolander OP, Kojima M, Serra JA, Koskinen P, Zhang J, Elo A, Mahonen AP, Street N, Bhalerao RP, Paulin L, Auvinen P, Sakakibara H, Helariutta Y (2016) Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr Biol 26:1990–1997
Jones AM (1998) Botany: auxin transport: down and out and up again. Science 282:2201–2202
Kamboj JS, Browning G, Quinlan JD, Blake PS, Baker DA (1997) Polar transport of H-3-IAA in apical shoot segments of different apple rootstocks. J Hortic Sci 72:773–780
Kramer EM (2004) PIN and AUX/LAX proteins: their role in auxin accumulation. Trends Plant Sci 9:578–582
Krecek P, Skupa P, Libus J, Naramoto S, Tejos R, Friml J, Zazimalova E (2009) The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol 10:11–11
Lachaud S, Bonnemain JL (1984) Seasonal variations in the polar-transport pathways and retention sites of [3H]indole-3-acetic acid in young branches of Fagus sylvatica L. Planta 161:207–215
Lee TI, Young RA (2000) Transcription of eukaryotic protein-coding genes. Annu Rev Genet 34:77–137
Li HL, Zhang H, Yu C, Ma L, Wang Y, Zhang XZ, Han ZH (2012) Possible roles of auxin and zeatin for initiating the dwarfing effect of M9 used as apple rootstock or interstock. Acta Physiol Plant 34:235–244
Liu HJ, Wang SF, Yu XB, Yu J, He XW, Zhang SL, Shou HX, Wu P (2005) ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 43:47–56
Lockard RG, Schneider GW (1981) Stock and scion growth relationships and the dwarfing mechanism in apple. Hortic Rev 315–375
Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Gene Dev 12:2175–2187
Marchant A, Bhalerao R, Casimiro I, Eklof J, Casero PJ, Bennett M, Sandberg G (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14:589–597
Martin MH, Goldsmith MHM, Goldsmith TH (1990) On polar auxin transport in plant-cells. J Math Biol 28:197–223
Maruyama-Nakashita A, Nakamura Y, Watanabe-Takahashi A, Inoue E, Yamaya T, Takahashi H (2005) Identification of a novel cis-acting element conferring sulfur deficiency response in Arabidopsis roots. Plant J 42:305–314
Matsunaga KKS, Cullen NP, Tomescu AMF (2017) Vascularization of the Selaginella rhizophore: anatomical fingerprints of polar auxin transport with implications for the deep fossil record. New Phytol 216:419–428
Moyle R, Schrader J, Stenberg A, Olsson O, Saxena S, Sandberg G, Bhalerao RP (2002) Environmental and auxin regulation of wood formation involves members of the Aux/IAA gene family in hybrid Aspen. Plant J 31:675–685
Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y (1991) Requirement of the auxin polar transport-system in early stages of Arabidopsis floral bud formation. Plant Cell 3:677–684
Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Wisniewska J, Tadele Z, Kubes M, Covanova M, Dhonukshe P, Skupa P, Benkova E, Perry L, Krecek P, Lee OR, Fink GR, Geisler M, Murphy AS, Luschnig C, Zazimalova E, Friml J (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312:914–918
Rathinasabapathi B, Mccue KF, Gage DA, Hanson AD (1994) Metabolic engineering of glycine betaine synthesis: plant betaine aldehyde dehydrogenases lacking typical transit peptides are targeted to tobacco chloroplasts where they confer betaine aldehyde resistance. Planta 193:155–162
Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C (2003) Regulation of phyllotaxis by polar auxin transport. Nature 426:255–260
Rigas S, Ditengou FA, Ljung K, Daras G, Tietz O, Palme K, Hatzopoulos P (2013) Root gravitropism and root hair development constitute coupled developmental responses regulated by auxin homeostasis in the arabidopsis root apex. New Phytol 197:1130–1141
Rocio Oliveros-Valenzuela M, Reyes D, Sanchez-Bravo J, Acosta M, Nicolas C (2007) The expression of genes coding for auxin carriers in different tissues and along the organ can explain variations in auxin transport and the growth pattern in etiolated lupin hypocotyls. Planta 227:133–142
Rolland-Lagan AG, Prusinkiewicz P (2005) Reviewing models of auxin canalization in the context of leaf vein pattern formation in Arabidopsis. Plant J 44:854–865
Sachs T (1981) The control of the patterned differentiation of vascular tissues. Adv Bot Res 9:151–262
Sachs T (1991) Cell polarity and tissue patterning in plants. Development 113:83–93
Santos A, Ribeiro R, Crespi AL (2004) Sweet cherry (Prunus avium) growth is mostly affected by rootstock and much less by budding height. N Z J Crop Hortic Sci 32:309–318
Santos F, Teale W, Fleck C, Volpers M, Ruperti B, Palme K (2010) Modelling polar auxin transport in developmental patterning. Plant Biol 12:3–14
Schrader J, Baba K, May ST, Palme K, Bennett M, Bhalerao RP, Sandberg G (2003) Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc Natl Acad Sci USA 100:10096–10101
Song C, Zhang D, Zhang J, Zheng L, Zhao C, Ma J, An N, Han M (2016) Expression analysis of key auxin synthesis, transport, and metabolism genes in different young dwarfing apple trees. Acta Physiol Plant 38:1–15
Soumelidou K, Morris DA, Battey NH, Barnett JR, John P (1994) Auxin transport capacity in relation to the dwarfing effect of apple rootstocks. J Hortic Sci 69:719–725
Sureshkumar M, Lee CK (2009) Biocatalytic reactions in hydrophobic ionic liquids. J Mol Catal B 60:1–12
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tanaka H, Dhonukshe P, Brewer PB, Friml J (2006) Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol Life Sci 63:2738–2754
Tsuda E, Yang H, Nishimura T, Uehara Y, Sakai T, Furutani M, Koshiba T, Hirose M, Nozaki H, Murphy AS, Hayashi K (2011) Alkoxy-auxins are selective inhibitors of auxin transport mediated by PIN, ABCB, and AUX1 transporters. J Biol Chem 286:2354–2364
Tuominen H, Puech L, Fink S, Sundberg B (1997) A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiol 115:577–585
Uggla C, Mellerowicz EJ, Sundberg B (1998) Indole-3-acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiol 117:113–121
van Hooijdonk B, Woolley D, Warrington I, Tustin S (2011) Rootstocks modify scion architecture, endogenous hormones, and root growth of newly grafted ‘Royal Gala’ apple trees. J Am Soc Hortic Sci 136:93–102
Venema JH, Dijk BE, Bax JM, van Hasselt PR, Elzenga JTM (2008) Grafting tomato (Solanum lycopersicum) onto the rootstock of a high-altitude accession of Solanum habrochaites improves suboptimal-temperature tolerance. Environ Exp Bot 63:359–367
Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J (2000) PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Dev 127:5157–5165
Wang J, Hu H, Wang G, Li J, Chen J, Wu P (2009) Expression of PIN genes in rice (Oryza sativa L.): tissue specificity and regulation by hormones. Mol Plant 2:823–831
Webster AD (1995) Rootstock and interstock effects on deciduous fruit tree vigour, precocity, and yield productivity. N Z J Crop Hortic Sci 23:373–382
Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312:883–883
Xu M, Zhu L, Shou HX, Wu P (2005) A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol 46:1674–1681
Yang Y, Li R, Qi M (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22:543–551
Zhang Y, Cheng J, Han Z, Xu X, Li T (2005) Comparison of methods for total RNA isolation from Malus Xiaojinensis and cDNA LD-PCR amplification. Biotechnol Inf 13:50–53
Zhang H, An HS, Wang Y, Zhang XZ, Han ZH (2015) Low expression of PIN gene family members is involved in triggering the dwarfing effect in M9 interstem but not in M9 rootstock apple trees. Acta Physiol Plant 37:1–18
Zhou DX, Yin K, Xu ZH, Xue HW (2003) Effect of polar auxin transport on rice root development. Acta Bot Sin 45:1421–1427
Zou C, Sun K, Mackaluso JD, Seddon AE, Jin R, Thomashow MF, Shiu SH (2011) Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc Natl Acad Sci USA 108:14992–14997
Acknowledgements
This work was supported by Beijing Municipal Education Commission (CEFF-PXM2017_014207_000043), the Earmarked Fund for China Agriculture Research System (CARS-27), and Key Laboratory of Biology and Genetic Improvement of Horticultural Crop (Nutrition and Physiology), Ministry of Agriculture.
Author information
Authors and Affiliations
Contributions
ZG designed and conducted the experiments, analyzed the data, accomplished pictures and wrote the manuscript. YW, TW, and XX contributed in design of the experiments. XZ designed the experiments and finalized the manuscript. ZH conceived and designed the experiments, and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gan, Z., Wang, Y., Wu, T. et al. MdPIN1b encodes a putative auxin efflux carrier and has different expression patterns in BC and M9 apple rootstocks. Plant Mol Biol 96, 353–365 (2018). https://doi.org/10.1007/s11103-018-0700-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-018-0700-6