Abstract
Key message
A p-coumaroyl CoA 2′-hydroxylase responsible for the formation of coumarin lactone ring was identified from Peucedanum praeruptorum Dunn and functionally characterized in vitro.
Abstract
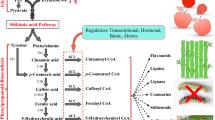
Coumarins are important plant secondary metabolites with a variety of biological activities. Ortho-hydroxylation of cinnamates leads to the formation of coumarin lactone ring and is generally thought to be a key step in coumarin biosynthesis. However, ortho-hydroxylases, especially p-coumaroyl CoA 2′-hydroxylase (C2′H) responsible for the biosynthesis of the most common coumarin skeleton, have received insufficient attention. Here, a putative ortho-hydroxylase PpC2′H was isolated from P. praeruptorum Dunn, a traditional Chinese medicinal herb rich in coumarins. Expression profile indicated that PpC2′H exhibited the highest transcript level in roots and could be up-regulated by MeJA elicitation. Subcellular localization of PpC2′H was demonstrated to be cytosol in planta. In order to functionally characterize PpC2′H, the purified recombinant protein was incubated with various potential substrates. HPLC-ESI-MS analysis indicated that PpC2′H catalyzed the conversion of p-coumaroyl CoA into hydroxylated intermediate, which then underwent spontaneous lactonization to generate umbelliferone. Our data also showed that light would promote the spontaneous process. In addition, based on homology modeling and site-directed mutagenesis, amino acid residues Phe-130, Lys-141, Asn-207, His-224, Asp-226, His-282 and Phe-298 were verified essential for enzymatic activity. These findings provide insight into structure–function relationship of this pivotal ortho-hydroxylase and also contribute to elucidating the biosynthetic mechanism of coumarin skeleton.








Similar content being viewed by others
References
Bai Y, Li D, Zhou T, Qin N, Li Z, Yu Z, Hua H (2016) Coumarins from the roots of Angelica dahurica with antioxidant and antiproliferative activities. J Funct Foods 20:453–462. doi:10.1016/j.jff.2015.11.018
Beuerle T, Pichersky E (2002) Enzymatic synthesis and purification of aromatic coenzyme a esters. Anal Biochem 302:305–312. doi:10.1006/abio.2001.5574
Bourgaud F, Hehn A, Larbat R, Doerper S, Gontier E, Kellner S, Matern U (2006) Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem Rev 5:293–308. doi:10.1007/s11101-006-9040-2
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1006/abio.1976.9999
Bugg TDH (2003) Dioxygenase enzymes: catalytic mechanisms and chemical models. Tetrahedron 59:7075–7101. doi:10.1016/s0040-4020(03)00944-x
Endler A, Martens S, Wellmann F, Matern U (2008) Unusually divergent 4-coumarate:CoA-ligases from Ruta graveolens L. Plant Mol Biol 67:335–346. doi:10.1007/s11103-008-9323-7
Ferrer JL, Austin MB, Stewart C, Noel JP (2008) Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem 46:356–370. doi:10.1016/j.plaphy.2007.12.009
Figueiras TS, Neves-Petersen MT, Petersen SB (2011) Activation energy of light induced isomerization of resveratrol. J Fluoresc 21:1897–1906. doi:10.1007/s10895-011-0886-3
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Bioi 40:347–369. doi:10.1146/annurev.pp.40.060189.002023
Hall BG (2013) Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol 30:1229–1235. doi:10.1093/molbev/mst012
Haskins FA, Williams LG, Gorz HJ (1964) Light-induced trans to cis conversion of β-d-glucosyl o-hydroxycinnamic acid in Melilotus alba leaves. Plant Physiol 39:777–781. doi:10.1104/pp.39.5.777
Kai K, Mizutani M, Kawamura N, Yamamoto R, Tamai M, Yamaguchi H, Sakata K, Shimizu B (2008) Scopoletin is biosynthesized via ortho-hydroxylation of feruloyl CoA by a 2-oxoglutarate-dependent dioxygenase in Arabidopsis thaliana. Plant J 55:989–999. doi:10.1111/j.1365-313X.2008.03568.x
Kal S, Que L (2017) Dioxygen activation by nonheme iron enzymes with the 2-His-1-carboxylate facial triad that generate high-valent oxoiron oxidants. J Biol Inorg Chem 22:339–365. doi:10.1007/s00775-016-1431-2
Kawai Y, Ono E, Mizutani M (2014) Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. Plant J 78:328–343. doi:10.1111/tpj.12479
Keating GJ, O’Kennedy R (1997) The chemistry and occurrence of coumarins. In: O’Kennedy R, Thornes RD (eds) Coumarins: biology, applications and mode of action. Wiley, Chichester, pp 23–66
Kim JH, Kim JK, Ahn EK, Ko HJ, Cho YR, Lee CH, Kim YK, Bae GU, Oh JS, Seo DW (2015) Marmesin is a novel angiogenesis inhibitor: regulatory effect and molecular mechanism on endothelial cell fate and angiogenesis. Cancer Lett 369:323–330. doi:10.1016/j.canlet.2015.09.021
Kong L, Li Y, Min Z, Li X, Zhu T (1996) Coumarins from Peucedanum praeruptorum. Phytochemistry 41:1423–1426. doi:10.1016/0031-9422(95)00783-0
Kühnl T, Koch U, Heller W, Wellmann E (1989) Elicitor induced S-adenosyl-l-methionine: caffeoyl-CoA 3-O-methyltransferase from carrot cell suspension cultures. Plant Sci 60:21–25. doi:10.1016/0168-9452(89)90039-3
Liu T, Yao R, Zhao Y, Xu S, Huang C, Luo J, Kong L (2017) Cloning, functional characterization and site-directed mutagenesis of 4-coumarate: coenzyme A ligase (4CL) involved in coumarin biosynthesis in Peucedanum praeruptorum Dunn. Front Plant Sci 8:4. doi:10.3389/fpls.2017.00004
Lukacin R, Britsch L (1997) Identification of strictly conserved histidine and arginine residues as part of the active site in Petunia hybrida flavanone 3β-hydroxylase. Eur J Biochem 249:748–757. doi:10.1111/j.1432-1033.1997.t01-2-00748.x
Markolovic S, Wilkins SE, Schofield CJ (2015) Protein hydroxylation catalyzed by 2-oxoglutarate-dependent oxygenases. J Biol Chem 290:20712–20722. doi:10.1074/jbc.R115.662627
Matern U (1991) Coumarins and other phenylpropanoid compounds in the defense response of plant cells. Planta Med 57:S15-S20. doi:10.1055/s-2006-960224
Matsumoto S, Mizutani M, Sakata K, Shimizu B (2012) Molecular cloning and functional analysis of the ortho-hydroxylases of p-coumaroyl coenzyme A/feruloyl coenzyme A involved in formation of umbelliferone and scopoletin in sweet potato, Ipomoea batatas (L.) Lam. Phytochemistry 74:49–57. doi:10.1016/j.phytochem.2011.11.009
Metternich JB, Gilmour R (2015) A bio-inspired, catalytic E → Z isomerization of activated olefins. J Am Chem Soc 137:11254–11257. doi:10.1021/jacs.5b07136
Metternich JB, Gilmour R (2016) One photocatalyst, n activation modes strategy for cascade catalysis: emulating coumarin biosynthesis with (-)-riboflavin. J Am Chem Soc 138:1040–1045. doi:10.1021/jacs.5b12081
Murray RD (1989) Coumarins. Nat Prod Rep 6:591–624
Ojala T, Remes S, Haansuu P, Vuorela H, Hiltunen R, Haahtela K, Vuorela P (2000) Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J Ethnopharmacol 73:299–305. doi:10.1016/S0378-8741(00)00279-8
Roselli S, Olry A, Vautrin S, Coriton O, Ritchie D, Galati G, Navrot N, Krieger C, Vialart G, Berges H, Bourgaud F, Hehn A (2016) A bacterial artificial chromosome (BAC) genomic approach reveals partial clustering of the furanocoumarin pathway genes in parsnip. Plant J 89:1119–1132. doi:10.1111/tpj.13450
Schinkovitz A, Gibbons S, Stavri M, Cocksedge MJ, Bucar F (2003) Ostruthin: an antimycobacterial coumarin from the roots of Peucedanum ostruthium. Planta Med 69:369–371. doi:10.1055/s-2003-38876
Schlucking K, Edel KH, Koster P, Drerup MM, Eckert C, Steinhorst L, Waadt R, Batistic O, Kudla J (2013) A new β-estradiol-inducible vector set that facilitates easy construction and efficient expression of transgenes reveals CBL3-dependent cytoplasm to tonoplast translocation of CIPK5. Mol Plant 6:1814–1829. doi:10.1093/mp/sst065
Schmid NB, Giehl RF, Doll S, Mock HP, Strehmel N, Scheel D, Kong X, Hider RC, von Wiren N (2014) Feruloyl-CoA 6′-hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol 164:160–172. doi:10.1104/pp.113.228544
Schmittgen TD (2006) Quantitative gene expression by real-time PCR: a complete protocol. In: Dorak MT (ed) Real-time PCR. Taylor and Francis Press, New York, pp 127–137
Shao M (2010) Pharmacopoeia of the People’s Republic of China (Part I). Chemical Industry Press, Beijing
Shimizu B (2014) 2-Oxoglutarate-dependent dioxygenases in the biosynthesis of simple coumarins. Front Plant Sci 5:549. doi:10.3389/fpls.2014.00549
Sun X, Zhou D, Kandavelu P, Zhang H, Yuan Q, Wang BC, Rose J, Yan Y (2015) Structural insights into substrate specificity of feruloyl-CoA 6′-hydroxylase from Arabidopsis thaliana. Sci Rep 5:10355. doi:10.1038/srep10355
Teutsch HG, Hasenfratz MP, Lesot A, Stoltz C, Garnier JM, Jeltsch JM, Durst F, Werck-Reichhart D (1993) Isolation and sequence of a cDNA encoding the Jerusalem artichoke cinnamate 4-hydroxylase, a major plant cytochrome P450 involved in the general phenylpropanoid pathway. Proc Natl Acad Sci USA 90:4102–4106
Vialart G, Hehn A, Olry A, Ito K, Krieger C, Larbat R, Paris C, Shimizu B, Sugimoto Y, Mizutani M, Bourgaud F (2012) A 2-oxoglutarate-dependent dioxygenase from Ruta graveolens L. exhibits p-coumaroyl CoA 2′-hydroxylase activity (C2′H): a missing step in the synthesis of umbelliferone in plants. Plant J 70:460–470. doi:10.1111/j.1365-313X.2011.04879.x
Weise NJ, Parmeggiani F, Ahmed ST, Turner NJ (2015) The bacterial ammonia lyase EncP: a tunable biocatalyst for the synthesis of unnatural amino acids. J Am Chem Soc 137:12977–12983. doi:10.1021/jacs.5b07326
Witaicenis A, Seito LN, da Silveira Chagas A, de Almeida LD Jr, Luchini AC, Rodrigues-Orsi P, Cestari SH, Di Stasi LC (2014) Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 21:240–246. doi:10.1016/j.phymed.2013.09.001
Wu JY, Fong WF, Zhang JX, Leung CH, Kwong HL, Yang MS, Li D, Cheung HY (2003) Reversal of multidrug resistance in cancer cells by pyranocoumarins isolated from Radix Peucedani. Eur J Pharmacol 473:9–17. doi:10.1016/S0014-2999(03)01946-0
Wu FH, Shen SC, Lee LY, Lee SH, Chan MT, Lin CS (2009) Tape-Arabidopsis sandwich: a simpler Arabidopsis protoplast isolation method. Plant Methods 5:16. doi:10.1186/1746-4811-5-16
Zhao Y, Liu T, Luo J, Zhang Q, Xu S, Han C, Xu J, Chen M, Chen Y, Kong L (2015) Integration of a decrescent transcriptome and metabolomics dataset of Peucedanum praeruptorum to investigate the CYP450 and MDR genes involved in coumarins biosynthesis and transport. Front Plant Sci 6:996. doi:10.3389/fpls.2015.00996
Zhao Y, Luo J, Xu S, Wang W, Liu T, Han C, Chen Y, Kong L (2016a) Selection of reference genes for gene expression normalization in Peucedanum praeruptorum Dunn under abiotic stresses, hormone treatments and different tissues. PLoS ONE 11:e0152356. doi:10.1371/journal.pone.0152356
Zhao Y, Wang N, Zeng Z, Xu S, Huang C, Wang W, Liu T, Luo J, Kong L (2016b) Cloning, functional characterization, and catalytic mechanism of a bergaptol O-methyltransferase from Peucedanum praeruptorum Dunn. Front Plant Sci 7:722. doi:10.3389/fpls.2016.00722
Zobel AM (1997) Coumarins in fruits and vegetables. In: Tomas-Barberan FAA, Robins RJ (eds) Phytochemistry of fruits and vegetables. Clarendon Press, Oxford, pp 173–203
Acknowledgements
This research was supported in part by the Natural Science Fund in Jiangsu Province (BK20170736), China Postdoctoral Science Foundation (1600020005), the National Natural Science Foundation of China (81430092), the Program for New Century Excellent Talents in University (NCET-2013-1035), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_15R63), and the Ph.D. Programs Foundation of Ministry of Education of China (20120096130002). We also thank the Cellular and Molecular Biology Center of China Pharmaceutical University for assistance with confocal microscopy work and we are grateful to Xiao-Nan Ma for her technical help.
Author information
Authors and Affiliations
Contributions
RY, YZ, JL and LK conceived and designed the work; RY, TL, YZ, SX and ZS performed the experiments; RY and CH interpreted and analyzed the data; RY wrote the paper; RY, YZ, TL, JL and LK revised the paper critically. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yao, R., Zhao, Y., Liu, T. et al. Identification and functional characterization of a p-coumaroyl CoA 2′-hydroxylase involved in the biosynthesis of coumarin skeleton from Peucedanum praeruptorum Dunn. Plant Mol Biol 95, 199–213 (2017). https://doi.org/10.1007/s11103-017-0650-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-017-0650-4