Abstract
Key message
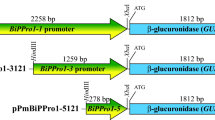
We demonstrate that RNAs of StBEL11 and StBEL29 are phloem-mobile and function antagonistically to the growth-promoting characteristics of StBEL5 in potato. Both these RNAs appear to inhibit tuber growth by repressing the activity of target genes of StBEL5 in potato. Moreover, upstream sequence driving GUS expression in transgenic potato lines demonstrated that both StBEL11 and -29 promoter activity is robust in leaf veins, petioles, stems, and vascular tissues and induced by short days in leaves and stolons. Steady-state levels of their mRNAs were also enhanced by short-day conditions in selective organs.
Abstract
There are thirteen functional BEL1-like genes in potato that encode for a family of transcription factors (TF) ubiquitous in the plant kingdom. These BEL1 TFs work in tandem with KNOTTED1-types to regulate the expression of numerous target genes involved in hormone metabolism and growth processes. One of the StBELs, StBEL5, functions as a long-distance mRNA signal that is transcribed in leaves and moves into roots and stolons to stimulate growth. The two most closely related StBELs to StBEL5 are StBEL11 and -29. Together these three genes make up more than 70% of all StBEL transcripts present throughout the potato plant. They share a number of common features, suggesting they may be co-functional in tuber development. Upstream sequence driving GUS expression in transgenic potato lines demonstrated that both StBEL11 and -29 promoter activity is robust in leaf veins, petioles, stems, and vascular tissues and induced by short-days in leaves and stolons. Steady-state levels of their mRNAs were also enhanced by short-day conditions in specific organs. Using a transgenic approach and heterografting experiments, we show that both these StBELs inhibit growth in correlation with the long distance transport of their mRNAs from leaves to roots and stolons, whereas suppression lines of these two RNAs exhibited enhanced tuber yields. In summary, our results indicate that the RNAs of StBEL11 and StBEL29 are phloem-mobile and function antagonistically to the growth-promoting characteristics of StBEL5. Both these RNAs appear to inhibit growth in tubers by repressing the activity of target genes of StBEL5.










Similar content being viewed by others
Abbreviations
- BELL:
-
BEL1-like transcription factor
- BRAVO:
-
Brassinosteroids at vascular and organizing center
- CLV:
-
Clavata
- FT:
-
Flowering locus T
- GA:
-
Gibberellin
- GAS:
-
Galactinol synthase
- GSP:
-
Gene-specific primers
- GUS:
-
β-Glucoronidase
- KNOX:
-
KNOTTED1-like Homeobox transcription factor
- LD:
-
Long-day
- OE:
-
Overexpression
- POTH1:
-
Potato homeobox 1
- PTB:
-
Polypyrimidine tract-binding protein
- QC:
-
Quiescent centre
- SAM:
-
Shoot apical meristem
- SD:
-
Short-day
- TALE:
-
Three amino acid loop extension family of proteins
- TF:
-
Transcription factor
- TFL1:
-
Terminal flower 1
- UTR:
-
Untranslated region
- WOX5:
-
WUSCHEL-RELATED HOMEOBOX5
- WUS:
-
WUSCHEL
References
Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309:1052–1056
Abelenda JA, Navarro C, Prat S (2014) Flowering and tuberization: a tale of two nightshades. Trends Plant Sci 19:115–122
Ayre BG, Blair JE, Turgeon R (2003) Functional and phylogenetic analyses of a conserved regulatory program in the phloem of minor veins. Plant Physiol 133:1229–1239
Banerjee AK, Chatterjee M, Yu Y, Suh SG, Miller WA, Hannapel DJ (2006a) Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell 18:3443–3457
Banerjee AK, Prat S, Hannapel DJ (2006b) Efficient production of transgenic potato (S. tuberosum L. ssp. andigena) plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 170:732–738
Banerjee AK, Lin T, Hannapel DJ (2009) Untranslated regions of a mobile transcript mediate RNA metabolism. Plant Physiol 151:1831–1843
Bhatt AM, Etchells JP, Canales C, Lagodienko A, Dickinson H (2004) VAAMANA-a BEL1-like homeodomain protein, interacts with KNOX proteins BP and STM and regulates inflorescence stem growth in Arabidopsis. Gene 328:103–111
Bhogale S, Mahajan AS, Natarajan B, Rajabhoj M, Thulasiram HV, Banerjee AK (2014) MicroRNA156: a potential graft-transmissible microRNA that modulates plant architecture and tuberization in Solanum tuberosum ssp. andigena. Plant Physiol 164:1011–1027
Bou-Torrent J, Martinez-Garcia JF, Garcia-Martinez JL, Prat S (2011) Gibberellin A1 metabolism contributes to the control of photoperiod-mediated tuberization in potato. PLoS One 6:e24458
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Burglin TR (1997) Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res 25:4173–4180
Byrne ME, Groover AT, Fontana JR, Martienssen RA (2003) Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELLRINGER. Development 130:3941–3950
Calderwood A, Kopriva S, Morris RJ (2016) Transcript abundance explains mRNA mobility data in Arabidopsis thaliana. Plant Cell 28:610–615
Chen H, Rosin FM, Hannapel DJ (2003) Interacting transcription factors from the three amino acid loop extension superclass regulate tuber formation. Plant Physiol 132:1391–1404
Chen H, Banerjee AK, Hannapel DJ (2004) The tandem complex of BEL and KNOX partners is required for transcriptional repression of ga20ox1. Plant J 38:276–284
Cho SK, Sharma P, Butler NM, Kang IH, Shah S, Rao AG, Hannapel DJ (2015) Polypyrimidine tract-binding proteins of potato mediate tuberization through an interaction with StBEL5 RNA. J Expt Bot 66:6835–6847
Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, Coupland G (2007) FT protein movement contributes to long distance signaling in floral induction of Arabidopsis. Science 316:1030–1033
Deeken R, Ache P, Kajahn I, Klinkenberg J, Bringmann G, Hedrich R (2008) Identification of Arabidopsis thaliana phloem RNAs provides a search criterion for phloem-based transcripts hidden in complex datasets of microarray experiments. Plant J 55:746–759
Dong YH, Yao JL, Atkinson RG, Putterill JJ, Morris BA, Gardner RC (2000) MDH1: an apple homeobox gene belonging to the BEL1 family. Plant Mol Biol 42:623–633
Fletcher JC (2002) Shoot and floral meristem maintenance in Arabidopsis. Annu Rev Plant Biol 53:45–66
González-Schain ND, Díaz-Mendoza M, Zurczak M, Suárez-López P (2012) Potato CONSTANS is involved in photoperiodic tuberization in a graft-transmissible manner. Plant J 70:678–690
Ham BK, Brandom JL, Xoconostle-Cázares B, Ringgold V, Lough TJ, Lucas WJ (2009) A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 21:197–215
Hannapel DJ, Sharma P, Lin T (2013) Phloem-mobile messenger RNAs and root development. Front Plant Sci 4:257
Hanzawa Y, Money T, Bradley D (2005) A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci USA 102:7748–7753
Hay A, Tsiantis M (2010) KNOX genes: versatile regulators of plant development and diversity. Development 137:3153–3165
Haywood V, Yu TS, Huang NC, Lucas WJ (2005) Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J 42:49–68
Huang NC, Jane WN, Chen J, Yu TS (2012) Arabidopsis CENTRORADIALIS homologue acts systemically to inhibit floral initiation in Arabidopsis. Plant J 72:175–184
Jaeger KE, Wigge PA (2007) FT protein acts as a long range signal in Arabidopsis. Curr Biol 17:1050–1054
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Kehr J, Buhtz A (2008) Long distance transport and movement of RNA through the phloem. J Exp Bot 59:85–92
Kim M, Canio W, Kessler S, Sinha N (2001) Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293:287–289
Kim D, Cho Y, Ryu H, Kim Y, Kim T, Hwang I (2013) BLH1 and KNAT3 modulate ABA responses during germination and early seedling development in Arabidopsis. Plant J 75:755–766
Kloosterman B, Navarro C, Bijsterbosch G, Lange T, Prat S, Visser RGF, Bachem CWB (2007) StGA2ox1 is induced prior to stolon swelling and controls GA levels during potato tuber development. Plant J 52:362–373
Kloosterman B, Abelenda JA, Gomez MdMC, Oortwijn M, de Boer JM, Kowitwanich K, Horvath BM, van Eck HJ, Smaczniak C, Prat S, Visser RGF, Bachem CWB (2013) Naturally occurring allele diversity allows potato cultivation in northern latitudes. Nature 495:246–250
Kumar R, Kushalappa K, Godt D, Pidkowich MS, Pastorelli S, Hepwort SR, Haughn GW (2007) The Arabidopsis BEL1-LIKE HOMEODOMAIN proteins SAW1 and SAW2 act redundantly to regulate KNOX expression spatially in leaf margins. Plant Cell 19:2719–2735
Li C, Gu M, Shi N, Zhang H, Yang X, Osman T, Liu Y, Wang H, Vatish M, Jackson S, Hong Y (2011) Mobile FT mRNA contributes to the systemic florigen signalling in floral induction. Sci Rep 1:73
Lifschitz E, Ayre BG, Eshed Y (2014) Florigen and anti-florigen: a systemic mechanism for coordinating growth and termination in flowering plants. Front Plant Sci 5:465. doi:10.3389/fpls.2014.00465
Lin T, Sharma P, Gonzalez DH, Viola IL, Hannapel DJ (2013) The impact of the long-distance transport of a BEL1-like mRNA on development. Plant Physiol 161:760–772
Lin T, Lashbrook CC, Cho SK, Butler NM, Sharma P, Muppirala U, Severin AJ, Hannapel DJ (2015) Transcriptional analysis of phloem-associated cells of potato. BMC Genom 16:665. doi:10.1186/s12864-015-1844-2
Livak KJ, Schmittgen (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C (T)) method. Methods 25:402–408
Lu KJ, Huang NC, Liu YS, Lu CA, Yu TS (2012) Long-distance movement of Arabidopsis FLOWERING LOCUS T RNA participates in systemic floral regulation. RNA Biol 9(5):653–662
Machackova I, Konstantinova TN, Seergeva LI, Lozhnikova VN, Golyanovskaya SA, Dudko ND, Eder J, Aksenova NP (1998) Photoperiodic control of growth, development and phytohormone balance in Solanum tuberosum. Physiol Plant 102:272–278
Mahajan A, Bhogle S, Kang II Ho, Hannapel DJ, Banerjee AK (2012) The mRNA of a Knotted1-like transcription factor of potato is phloem mobile. Plant Mol Biol 79:595–608
Martin A, Adam H, Díaz-Mendoza M, Zurczak M, González-Schain ND, Suárez-López P (2009) Graft-transmissible induction of potato tuberization by the microRNA miR172. Development 136:2873–2881
Mathieu J, Warthmann N, Küttner F, Schmid M (2007) Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol 17:1055–1060
Mignery GA, Pikaard CS, Hannapel DJ, Park WD (1984) Isolation and sequence analysis of cDNAs for the major potato tuber protein, patatin. Nucleic Acids Res 12:7987–8000
Mukherjee K, Brocchieri L, Burglin TR (2009) A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol Biol Evol 26:2775–2794
Navarro C, Abelenda JA, Cruz-Oró E, Cuéllar CA, Tamaki S, Silva J, Shimamoto K, Prat S (2011) Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 478:119–122
Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A, Tomita Y, Dohi K, Mori M, Araki T (2008) Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol 49:1645–1658
Notaguchi M, Wolf S, Lucas WJ (2012) Phloem-mobile Aux/IAA transcripts target to the root tip and modify root architecture. J Int Plant Biol 54:760–772
Notaguchi M, Higashiyama T, Suzuki T (2015) Identification of mRNAs that move over long distances using an RNA-Seq analysis of Arabidopsis/Nicotiana benthamiana heterografts. Plant Cell Physiol 56:311–321
Omid A, Keilin T, Glass A, Leshkowitz D, Wolf S (2007) Characterization of phloem-sap transcription profile in melon plants. J Exp Bot 58:3645–3656
Passner M, Ryoo HD, Shen L, Mann RS, Aggarwal AK (1999) Structure of DNA-bound ultrabithorax-extradenticle homeodomain complex. Nature 397:714–719
Ragni L, Belles-Boix E, Gunl M, Pautot V (2008) Interaction of KNAT6 and KNAT2 with BREVIPEDICELLUS and PENNYWISE in Arabidopsis inflorescences. Plant Cell 20:888–900
Ray A, Robinson-Beer K, Ray S, Baker SC, Lang JD, Preus D, Milligan SB, Gasser CS (1994) Arabidopsis floral homeotic gene BELL (BELI) controls ovule development through negative regulation of AGAMOUS gene. Proc Nat Acad Sci USA 97:5761–5765
Reiser L, Modrusan Z, Margossian L, Samach A, Ohad N, Haughn GW, Fischer RL (1995) The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell 83:735–742
Roeder AH, Ferrandiz C, Yanofsky MF (2003) The role of the REPLUMLESS homeodomain protein in patterning the Arabidopsis fruit. Curr Biol 13:1630–1635
Roumeliotis E, Visser RG, Bachem CW (2012) A crosstalk of auxin and GA during tuber development. Plant Signal Behav 7:1360–1363
Rutjens B, Bao D, van Eck-Stouten E, Brand M, Smeekens S, Proveniers M (2009) Shoot apical meristem function in Arabidopsis requires the combined activities of three BEL1-like homeodomain proteins. Plant J 5:641–665
Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100:635–644
Shannon S, Meeks-Wagner DR (1991) A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3:877–892
Sharma P, Lin T, Grandellis C, Yu M, Hannapel DJ (2014) The BEL1-like family of transcription factors in potato. J Expt Bot 65:709–723
Sharma P, Lin T, Hannapel DJ (2016) Targets of the StBEL5 transcription factor include the FT ortholog StSP6A. Plant Physiol 170:310–324
Staneloni RJ, Rodriguez-Batiller MJ, Legisa D, Scarpin MR, Agalou A, Cerdán PD, Meijer AH, Ouwerkerk PB, Casal JJ (2009) Bell-like homeodomain selectively regulates the high-irradiance response of phytochrome A. Proc Natl Acad Sci USA 106:13624–13629
Sun XD, Feng ZH, Meng LS, Zhu J, Geitmann A (2013) Arabidopsis ASL11/LBD15 is involved in shoot apical meristem development and regulates WUS expression. Planta 237:1367–1378
Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309:1056–1059
Xiang C, Han P, Lutziger I, Wang K, Oliver DJ (1999) A mini binary vector series for plant transformation. Plant Mol Biol 40:711–717
Xu X, van Lammeren AAM, Vermeer E, Vreugdenhil D (1998a) The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol 117:575–584
Xu X, Vreugdenhil D, van Lammeren AAM (1998b) Cell division and cell enlargement during potato tuber formation. J Exp Bot 49:573–582
Xu X et al (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–195
Yu Y, Lashbrook CC, Hannapel DJ (2007) Tissue integrity and RNA quality of laser microdissected phloem of potato. Planta 226:797–803
Žádníková P, Simon R (2014) How boundaries control plant development. Curr Opin Plant Biol 17:116–125
Acknowledgements
Banerjee lab members gratefully acknowledge the support and core funding from Indian Institute of Science Education and Research (IISER Pune), and Department of Science and Technology (DST), Govt. of India (Grant No. SR/SO/PS-28/2010). KK acknowledge the fellowship provided by the Department of Biotechnology (DBT), India. We also thank Dr. M. M. Jana and Mr. Nitish Lahigude of IISER Pune for their help in plant maintenance in growth chambers. Contributions of the Hannapel lab were supported by the National Science Foundation Plant Genome Research Program award no. DBI-0820659.
Authors’ contributions
TG, PS and KK have generated all the constructs, transgenic lines and have conducted the experiments. DH and AKB have conceived the idea and written the manuscript and all other authors have approved it.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Additional information
Tejashree H. Ghate and Pooja Sharma have contributed equally as first authors on this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghate, T.H., Sharma, P., Kondhare, K.R. et al. The mobile RNAs, StBEL11 and StBEL29, suppress growth of tubers in potato. Plant Mol Biol 93, 563–578 (2017). https://doi.org/10.1007/s11103-016-0582-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-016-0582-4