Abstract
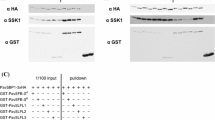
Self-incompatible solanaceous species possess the S-RNase and SLF (S-locus F-box) genes at the highly polymorphic S-locus, and their products mediate S-haplotype-specific rejection of pollen tubes in the style. After a pollen tube grows into the style, the S-RNases produced in the style are taken up; however, only self S-RNase (product of the matching S-haplotype) can inhibit the subsequent growth of the pollen tube. Based on the finding that non-self interactions between PiSLF (Petunia inflata SLF) and S-RNase are stronger than self-interactions, and based on the biochemical properties of PiSLF, we previously proposed that a PiSLF preferentially interacts with its non-self S-RNases to mediate their ubiquitination and degradation, thereby only allowing self S-RNase to exert its cytotoxic function. We further divided PiSLF into three potential Functional Domains (FDs), FD1-FD3, based on sequence comparison of PiSLF and PiSLF-like proteins, and based on S-RNase-binding properties of these proteins and various truncated forms of PiSLF2 (S 2 allelic variant of PiSLF). In this work, we examined the in vivo function of FD2, which we proposed to be responsible for strong, general interactions between PiSLF and S-RNase. We swapped FD2 of PiSLF2 with the corresponding region of PiSLFLb-S2 (S 2 allelic variant of a PiSLF-like protein), and expressed GFP-fused chimeric proteins, named b-2-b and 2-b-2, in S 2 S 3 transgenic plants. We showed that neither chimeric protein retained the SI function of PiSLF2, suggesting that FD2 is necessary, but not sufficient, for the function of PiSLF. Moreover, since we previously found that b-2-b and 2-b-2 only interacted with S3-RNase ~50 and ~30%, respectively, as strongly as did PiSLF2 in vitro, their inability to function as PiSLF2 is also consistent with our model predicating on strong interaction between a PiSLF and its non-self S-RNases as part of the biochemical basis for S-haplotype-specific rejection of pollen tubes.








Similar content being viewed by others
Abbreviations
- FD:
-
Functional domain
- GFP:
-
Green fluorescent protein
- GST:
-
Glutathione-S-Transferase
- HMM:
-
Hidden Markov model
- NOS-ter:
-
Transcription termination signal of the nopaline synthase gene
- PP:
-
Posterior probability
- PPd:
-
Posterior probability of divergence
- PiSLF:
-
Petunia inflataS-locus F-box protein
- PiSLFL:
-
Petunia inflataS-locus F-box like protein
- pLAT52 :
-
The promoter of LAT52 of tomato
- PVDF:
-
Polyvinylidene difluoride
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- SCF:
-
Skp1 Cullin-1 F-box and Rbx1
- SI:
-
Self-incompatibility
- SLF:
-
S-locus F-box protein
- SLF :
-
S-locus F-box
- SR:
-
Specific region
References
Ai Y, Singh A, Coleman CE, Ioerger TR, Kheyr-Pour A, Kao T-h (1990) Self-incompatibility in Petunia inflata: isolation and characterization of cDNAs encoding three S-allele-associated proteins. Sex Plant Reprod 3:130–138
de Nettancourt D (2001) Incompatibility and incongruity in wild and cultivated plants. Springer, Berlin
Dowd PE, Coursol S, Skirpan A, Kao T-h, Gilroy S (2006) Petunia phospholipase C1 is involved in pollen tube growth. Plant Cell 18:1438–1453
Frankin-Tong V (2008) Self-incompatibility in flowering plants–evolution diversity, and mechanisms. Springer, Berlin
Goldraij A, Kondo K, Lee CB, Hancock CN, Sivaguru M, Vazquez-Santana S, Kim S, Phillips TE, Cruz-Garcia F, McClure B (2006) Compartmentalization of S-RNase and HT-B degradation in self-incompatible Nicotiana. Nature 439:805–810
Hershko A, Ciechanover A (1998) The ubiquitin system. Ann Rev Biochem 67:425–479
Holden MJ, Marty JA, Singh-Cundy A (2003) Pollination induced ethylene promotes the early phase of pollen tube growth in Petunia inflata. J Plant Physiol 160:261–269
Hua Z, Kao T-h (2006) Identification and characterization of components of a putative Petunia S-Locus F-Box-containing E3 ligase complex involved in S-RNase–based self-incompatibility. Plant Cell 18:2531–2553
Hua Z, Kao T-h (2008) Identification of major lysine residues of S3-RNase of Petunia inflata involved in ubiquitin-26S proteasome-mediated degradation in vitro. Plant J 54:1094–1104
Hua Z, Meng XY, Kao T-h (2007) Comparison of Petunia inflata S-locus F-box protein (Pi SLF) with Pi SLF-like proteins reveals its unique function in S-RNase-based self-incompatibility. Plant Cell 19:3593–3609
Hua Z, Fields A, Kao T-h (2008) Biochemical Models for S-RNase-Based Self-Incompatibility. Mol Plant 1:575–585
Kao T-h, Tsukamoto T (2004) The molecular and genetic bases of S-RNase-based self-incompatibility. Plant Cell 16:S72–S83
Lee H-S, Huang S, Kao T-h (1994) S proteins control rejection of incompatible pollen in Petunia inflata. Nature 367:560–563
Liu B, Morse D, Cappadocia M (2009) Compatible pollinations in Solanum chacoense decrease both S-RNase and S-RNase mRNA. PLoS ONE 4(6):e5774
Luu D-T, Qin X, Morse D, Cappadocia M (2000) S-RNase uptake by compatible pollen tubes in gametophytic self-incompatibility. Nature 407:649–651
McClure B (2009) Darwin’s foundation for investigating self-incompatibility and the progress toward a physiological model for S-RNase-based SI. J Exp Bot 60:1069–1081
Meng XY, Hua Z, Wang N, Fields A, Dowd PE, Kao T-h (2009) Ectopic expression of S-RNase of Pentunia inflata in pollen results in its sequestration and non-cytotoxic function. Sex Plant Reprod 22:263–275
Moon J, Parry G, Estelle M (2004) The ubiquitin-proteasome pathway and plant development. Plant Cell 16:3181–3195
Qin X, Liu B, Soulard J, Morse D, Cappadocia M (2006) Style-by-style analysis of two sporadic self-compatible Solanum chacoense lines supports a primary role for S-RNases in determining pollen rejection thresholds. J Exp Bot 57:2001–2013
Sijacic P, Wang X, Skirpan AL, Wang Y, Dowd PE, McCubbin AG, Huang S, Kao T-h (2004) Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature 429:302–305
Skirpan AL, McCubbin AG, Ishimizu T, Wang X, Hu Y, Dowd PE, Ma H, Kao T-h (2001) Isolation and characterization of kinase interacting protein 1, a pollen protein that interacts with the kinase domain of PRK1, a receptor-like kinase of Petunia. Plant Physiol 126:1480–1492
Smalle J, Vierstra RD (2004) The ubiquitin 26S proteasome proteolytic pathway. Annu Rev Plant Biol 55:555–590
Twell D, Yamaguchi J, McCormick S (1990) Pollen-specific gene expression in transgenic plants: Coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109:705–713
Wang L, Dong L, Zhang Y, Zhang Y, Wu W, Deng X, Xue Y (2005) Genome-wide analysis of S-locus F-box-like genes in Arabidopsis thaliana. Plant Mol Biol 56:929–945
Zhang Y, Zhao Z, Xue Y (2009) Roles of proteolysis in plant self-incompatibility. Annu Rev Plant Biol 60:21–42
Acknowledgments
We thank Michael Goralczyk and Melinda Bothe for assistance in genomic DNA preparation, Jiong Wang for general laboratory help, and Anthony Omeis for greenhouse management. This work was supported by National Science Foundation grant IOS 08-43195 to T.-h.K.
Author information
Authors and Affiliations
Corresponding author
Additional information
Accession numbers
PiSLF1 (AY500390); PiSLF2 (AY500391); PiSLF3 (AY500392); PiSLFLa-S1 (EF614190); PiSLFLa-S2 (EF614189); PiSLFLb-S2 (EF614188); PiSLFLc-S1 (EF614191); PiSLFLd-S2 (EF614187); S1(S2)A113 (AY363970AY363971); S3A113 (AY363972); S1A134 (AY363973); S2A134 (AY363974); S3A134(AY363975).
Allison M. Fields and Ning Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fields, A.M., Wang, N., Hua, Z. et al. Functional characterization of two chimeric proteins between a Petunia inflata S-locus F-box protein, PiSLF2, and a PiSLF-like protein, PiSLFLb-S2 . Plant Mol Biol 74, 279–292 (2010). https://doi.org/10.1007/s11103-010-9672-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9672-x