Abstract
Purpose
Insulin sensitivity (Si) and its role in glucose intolerance of acromegaly has been extensively evaluated. However, data on insulin secretion is limited. We aimed to assess stimulated insulin secretion using an intravenous glucose tolerance test (IVGTT) in active acromegaly.
Methods
We performed an IVGTT in 25 patients with active acromegaly (13 normal glucose tolerance [NGT], 6 impaired glucose tolerance [IGT] and 6 diabetes mellitus [DM]) and 23 controls (8 lean NGT, 8 obese NGT and 7 obese IGT). Serum glucose and insulin were measured at 20 time points along the test to calculate Si and acute insulin response (AIRg). Medical treatment for acromegaly or diabetes was not allowed.
Results
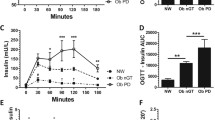
In acromegaly, patients with NGT had significantly (p for trend < 0.001) higher AIRg (3383 ± 1082 pmol*min/L) than IGT (1215 ± 1069) and DM (506 ± 600). AIRg was higher in NGT (4764 ± 1180 pmol*min/L) and IGT (3183 ± 3261) controls with obesity than NGT (p = 0.01) or IGT (p = 0.17) acromegaly. Si was not significantly lower in IGT (0.68 [0.37, 0.88] 106*L/pmol*min) and DM (0.60 [0.42, 0.84]) than in NGT (0.81 [0.58, 1.55]) patients with acromegaly. NGT (0.33 [0.30, 0.47] 106*L/pmol*min) and IGT (0.37 [0.21, 0.66]) controls with obesity had lower Si than NGT (p = 0.001) and IGT (p = 0.43) acromegaly.
Conclusion
We demonstrated that low insulin secretion is the main driver behind glucose intolerance in acromegaly. Compared to NGT and IGT controls with obesity, patients with NGT or IGT acromegaly had higher Si. Together, these findings suggest that impaired insulin secretion might be a specific mechanism for glucose intolerance in acromegaly.


Similar content being viewed by others
Data availability
Raw data are available from the corresponding author upon reasonable request.
References
Gadelha MR, Kasuki L, Lim DST, Fleseriu M (2019) Systemic complications of Acromegaly and the impact of the Current Treatment Landscape: an update. Endocr Rev 40:268–332. https://doi.org/10.1210/er.2018-00115
Melmed S, Kaiser UB, Lopes MB, Bertherat J, Syro LV, Raverot G, Reincke M, Johannsson G, Beckers A, Fleseriu M, Giustina A, Wass JAH, Ho KKY (2022) Clinical Biology of the Pituitary Adenoma. Endocr Rev 43:1003–1037. https://doi.org/10.1210/ENDREV/BNAC010
Fleseriu M, Langlois F, Lim DST, Varlamov EV, Melmed S (2022) Acromegaly: pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol 10:804–826. https://doi.org/10.1016/S2213-8587(22)00244-3
Giustina A, Barkhoudarian G, Beckers A, Ben-Shlomo A, Biermasz N, Biller B, Boguszewski C, Bolanowski M, Bollerslev J, Bonert V, Bronstein MD, Buchfelder M, Casanueva F, Chanson P, Clemmons D, Fleseriu M, Formenti AM, Freda P, Gadelha M, Geer E, Gurnell M, Heaney AP, Ho KKY, Ioachimescu AG, Lamberts S, Laws E, Losa M, Maffei P, Mamelak A, Mercado M, Molitch M, Mortini P, Pereira AM, Petersenn S, Post K, Puig-Domingo M, Salvatori R, Samson SL, Shimon I, Strasburger C, Swearingen B, Trainer P, Vance ML, Wass J, Wierman ME, Yuen KCJ, Zatelli MC, Melmed S (2020) Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord 21:667–678. https://doi.org/10.1007/s11154-020-09588-z
Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, Bolanowski M, Bonert V, Bronstein MD, Casanueva FF, Clemmons D, Colao A, Ferone D, Fleseriu M, Frara S, Gadelha MR, Ghigo E, Gurnell M, Heaney AP, Ho K, Ioachimescu A, Katznelson L, Kelestimur F, Kopchick J, Krsek M, Lamberts S, Losa M, Luger A, Maffei P, Marazuela M, Mazziotti G, Mercado M, Mortini P, Neggers S, Pereira AM, Petersenn S, Puig-Domingo M, Salvatori R, Shimon I, Strasburger C, Tsagarakis S, van der Lely AJ, Wass J, Zatelli MC, Melmed S (2020) A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab 105:E937–E946. https://doi.org/10.1210/clinem/dgz096
Frara S, Maffezzoni F, Mazziotti G, Giustina A (2016) Current and emerging aspects of diabetes Mellitus in Acromegaly. Trends Endocrinol Metab 27:470–483. https://doi.org/10.1016/j.tem.2016.04.014
Albertelli M, Nazzari E, Dotto A, Grasso LF, Sciallero S, Pirchio R, Rebora A, Boschetti M, Pivonello R, Ricci Bitti S, Colao AAL, Ferone D (2021) Possible protective role of metformin therapy on colonic polyps in acromegaly: an exploratory cross-sectional study. Eur J Endocrinol 184:423–429. https://doi.org/10.1530/EJE-20-0795
Moustaki M, Paschou SA, Xekouki P, Kotsa K, Peppa M, Psaltopoulou T, Kalantaridou S, Vryonidou A (2023) Secondary diabetes mellitus in acromegaly. Endocrine 81:1–15. https://doi.org/10.1007/s12020-023-03339-1
Biagetti B, Aulinas A, Casteras A, Pérez-Hoyos S, Simó R (2021) HOMA-IR in acromegaly: a systematic review and meta-analysis. Pituitary 24:146–158. https://doi.org/10.1007/s11102-020-01092-6
Baldelli R, Battista C, Leonetti F, Ghiggi MR, Ribaudo MC, Paoloni A, D’Amico E, Ferretti E, Baratta R, Liuzzi A, Trischitta V, Tamburrano G (2003) Glucose homeostasis in acromegaly: effects of long-acting somatostatin analogues treatment. Clin Endocrinol (Oxf) 59:492–499. https://doi.org/10.1046/j.1365-2265.2003.01876.x
Battezzati A, Benedini S, Fattorini A, Losa M, Mortini P, Bertoli S, Lanzi R, Testolin G, Biolo G, Luzi L (2003) Insulin action on protein metabolism in acromegalic patients. Am J Physiol Endocrinol Metab 284:E823–E829. https://doi.org/10.1152/ajpendo.00020.2002
Moller N, Schmitz O, Joorgensen JO, Astrup J, Bak JF, Christensen SE, Alberti KG, Weeke J (1992) Basal- and insulin-stimulated substrate metabolism in patients with active acromegaly before and after adenomectomy. J Clin Endocrinol Metab 74:1012–1019
Alexopoulou O, Bex M, Kamenicky P, Mvoula AB, Chanson P, Maiter D (2014) Prevalence and risk factors of impaired glucose tolerance and diabetes mellitus at diagnosis of acromegaly: a study in 148 patients. Pituitary 17:81–89. https://doi.org/10.1007/s11102-013-0471-7
Wang Z, Gao L, Guo X, Feng C, Deng K, Lian W, Feng M, Bao X, Xing B (2019) Preoperative fasting C-Peptide acts as a promising predictor of improved glucose tolerance in patients with Acromegaly after Transsphenoidal surgery: a retrospective study of 64 cases from a large Pituitary Center in China. Front Endocrinol (Lausanne) 10:736. https://doi.org/10.3389/fendo.2019.00736
Shekhawat VS, Bhansali S, Dutta P, Mukherjee KK, Vaiphei K, Kochhar R, Sinha SK, Sachdeva N, Kurpad AV, Bhat K, Mudaliar S, Bhansali A (2019) Glucose-dependent Insulinotropic polypeptide (GIP) resistance and β-cell dysfunction contribute to Hyperglycaemia in Acromegaly. Sci Rep 9:5646. https://doi.org/10.1038/s41598-019-41887-7
Niculescu D, Purice M, Lichiardopol R, Coculescu M (2010) Both insulin resistance and insulin secretion are involved in the pre-diabetes of Acromegaly. Acta Endocrinol 6:35–42. https://doi.org/10.4183/aeb.2010.35
Kinoshita Y, Fujii H, Takeshita A, Taguchi M, Miyakawa M, Oyama K, Yamada S, Takeuchi Y (2011) Impaired glucose metabolism in Japanese patients with acromegaly is restored after successful pituitary surgery if pancreatic {beta}-cell function is preserved. Eur J Endocrinol 164:467–473. https://doi.org/10.1530/EJE-10-1096
He W, Yan L, Wang M, Li Q, He M, Ma Z, Ye Z, Zhang Q, Zhang Y, Qiao N, Lu Y, Ye H, Lu B, Shou X, Zhao Y, Li Y, Li S, Zhang Z, Shen M, Wang Y (2019) Surgical outcomes and predictors of glucose metabolism alterations for growth hormone-secreting pituitary adenomas: a hospital-based study of 151 cases. Endocrine 63:27–35. https://doi.org/10.1007/s12020-018-1745-7
Niculescu DA, Dusceac R, Caragheorgheopol A, Popescu N, Poiana C (2019) Disposition Index in active acromegaly. Front Endocrinol (Lausanne) 10:637. https://doi.org/10.3389/fendo.2019.00637
Giustina A, Biermasz N, Casanueva FF, Fleseriu M, Mortini P, Strasburger C, van der Lely AJ, Wass J, Melmed S (2023) Consensus on criteria for acromegaly diagnosis and remission. https://doi.org/10.1007/s11102-023-01360-1. Acromegaly Consensus GroupPituitary
ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry M, Lou, Prahalad P, Pratley RE, Seley JJ, Stanton RC, Gabbay RA, on behalf of the American Diabetes Association (2023) 2. Classification and diagnosis of diabetes: standards of Care in Diabetes-2023. Diabetes Care 46:S19–S40. https://doi.org/10.2337/dc23-S002
Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL (2014) Assessment of pancreatic β-cell function: review of methods and clinical applications. Curr Diabetes Rev 10:2–42
Bunt JC, Krakoff J, Ortega E, Knowler WC, Bogardus C (2007) Acute insulin response is an independent predictor of type 2 diabetes mellitus in individuals with both normal fasting and 2-h plasma glucose concentrations. Diabetes Metab Res Rev 23:304–310. https://doi.org/10.1002/dmrr.686
Gastaldelli A (2022) Measuring and estimating insulin resistance in clinical and research settings. Obesity 30:1549–1563. https://doi.org/10.1002/oby.23503
Espinosa-de-Los-Monteros AL, Gonzalez B, Vargas G, Sosa E, Mercado M (2011) Clinical and biochemical characteristics of acromegalic patients with different abnormalities in glucose metabolism. Pituitary 14:231–235
Ciresi A, Amato MC, Pivonello R, Nazzari E, Grasso LF, Minuto F, Ferone D, Colao A, Giordano C (2013) The metabolic profile in active acromegaly is gender-specific. J Clin Endocrinol Metab 98:E51–E59. https://doi.org/10.1210/jc.2012-2896
Niculescu D, Purice M, Coculescu M (2013) Insulin-like growth factor-I correlates more closely than growth hormone with insulin resistance and glucose intolerance in patients with acromegaly. Pituitary 16:168–174. https://doi.org/10.1007/s11102-012-0396-6
Petrossians P, Daly AF, Natchev E, Maione L, Blijdorp K, Sahnoun-Fathallah M, Auriemma R, Diallo AM, Hulting AL, Ferone D, Hana V, Filipponi S, Sievers C, Nogueira C, Fajardo-Montañana C, Carvalho D, Hana V, Stalla GK, Jaffrain-Réa ML, Delemer B, Colao A, Brue T, Neggers SJCMM, Zacharieva S, Chanson P, Beckers A (2017) Acromegaly at diagnosis in 3173 patients from the Liège Acromegaly Survey (LAS) database. Endocr Relat Cancer 24:505–518. https://doi.org/10.1530/ERC-17-0253
Arosio M, Reimondo G, Malchiodi E, Berchialla P, Borraccino A, De Marinis L, Pivonello R, Grottoli S, Losa M, Cannavò S, Minuto F, Montini M, Bondanelli M, De Menis E, Martini C, Angeletti G, Velardo A, Peri A, Faustini-Fustini M, Tita P, Pigliaru F, Borretta G, Scaroni C, Bazzoni N, Bianchi A, Appetecchia M, Cavagnini F, Lombardi G, Ghigo E, Beck-Peccoz P, Colao A, Terzolo M, Italian Study Group of Acromegaly (2012) Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur J Endocrinol 167:189–198. https://doi.org/10.1530/EJE-12-0084
Mestron A, Webb SM, Astorga R, Benito P, Catala M, Gaztambide S, Gomez JM, Halperin I, Lucas-Morante T, Moreno B, Obiols G, de Pablos P, Paramo C, Pico A, Torres E, Varela C, Vazquez JA, Zamora J, Albareda M, Gilabert M (2004) Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol De Acromegalia, REA). Eur J Endocrinol 151:439–446
Wolf P, Dormoy A, Maione L, Salenave S, Young J, Kamenický P, Chanson P (2022) Impairment in insulin secretion without changes in insulin resistance explains hyperglycemia in patients with acromegaly treated with pasireotide LAR. Endocr Connect 11:e220296. https://doi.org/10.1530/EC-22-0296
Freda PU, Shen W, Reyes-Vidal CM, Geer EB, Arias-Mendoza F, Gallagher D, Heymsfield SB (2009) Skeletal muscle mass in acromegaly assessed by magnetic resonance imaging and dual-photon x-ray absorptiometry. J Clin Endocrinol Metab 94:2880–2886. https://doi.org/10.1210/jc.2009-0026
Ferraù F, Albani A, Ciresi A, Giordano C, Cannavò S (2018) Diabetes secondary to Acromegaly: Physiopathology, clinical features and effects of Treatment. Front Endocrinol (Lausanne) 9:358. https://doi.org/10.3389/fendo.2018.00358
Hauguel-Moreau M, Hergault H, Cazabat L, Pépin M, Beauchet A, Aïdan V, Ouadahi M, Josseran L, Hage M, Rodon C, Dubourg O, Massy Z, Mansencal N (2023) Prevalence of prediabetes and undiagnosed diabetes in a large urban middle-aged population: the CARVAR 92 cohort. Cardiovasc Diabetol 22:31. https://doi.org/10.1186/s12933-023-01761-3
Moody A, Cowley G, Ng Fat L, Mindell JS (2016) Social inequalities in prevalence of diagnosed and undiagnosed diabetes and impaired glucose regulation in participants in the health surveys for England series. BMJ Open 6:e010155. https://doi.org/10.1136/bmjopen-2015-010155
Bjarkø VV, Haug EB, Sørgjerd EP, Stene LC, Ruiz PL, Birkeland KI, Berg TJ, Gulseth HL, Iversen MM, Langhammer A, Åsvold BO (2022) Undiagnosed diabetes: prevalence and cardiovascular risk profile in a population-based study of 52,856 individuals. The HUNT study, Norway. Diabet Med 39:e14829. https://doi.org/10.1111/dme.14829
Yu H, Ho M, Liu X, Yang J, Chau PH, Fong DYT (2023) Incidence and temporal trends in type 2 diabetes by weight status: a systematic review and meta-analysis of prospective cohort studies. J Glob Health 13:04088. https://doi.org/10.7189/jogh.13.04088
Yu H, Ho M, Liu X, Yang J, Chau PH, Fong DYT (2022) Association of weight status and the risks of diabetes in adults: a systematic review and meta-analysis of prospective cohort studies. Int J Obes 46:1101–1113. https://doi.org/10.1038/s41366-022-01096-1
Wang S, Wu J, Wang N, Zeng L, Wu Y (2017) The role of growth hormone receptor in β cell function. Growth Horm IGF Res 36:30–35. https://doi.org/10.1016/j.ghir.2017.08.002
Acknowledgements
We want to thank Professor Maria Fleseriu, MD, for helpful discussion and advice for this manuscript.
Funding
This work supported by the 57404559/11.11.2019 Independent Research Grant from Pfizer and 28332/04.11.2013 Young Researches Grant from Carol Davila University of Medicine and Pharmacy.
Author information
Authors and Affiliations
Contributions
Study design: L.G.Z., D.A.N. and C.P. Data collection: L.G.Z., D.A.N., A.E.K., A.C., M.S., C.N.I., R.D. and I.F.B. Data interpretation: L.G.Z. and D.A.N. Writing: L.G.Z., D.A.N. and R.D. Critical review and approval of the final version: all authors.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the C. I. Parhon National Institute of Endocrinology Ethics Committee (No 9/27.03.2020).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaifu, L.G., Niculescu, D.A., Kremer, A.E. et al. Glucose intolerance in acromegaly is driven by low insulin secretion; results from an intravenous glucose tolerance test. Pituitary 27, 178–186 (2024). https://doi.org/10.1007/s11102-024-01386-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-024-01386-z