Abstract
Purpose
Pituitary adenomas are the most common tumor of the pituitary gland and comprise nearly 15% of all intracranial masses. These tumors are stratified into functional or silent categories based on their pattern of hormone expression and secretion. Preliminary evidence supports differential clinical outcomes between some functional pituitary adenoma (FPA) subtypes and silent pituitary adenoma (SPA) subtypes.
Methods
We collected and analyzed the medical records of all patients undergoing resection of SPAs or FPAs from a single high-volume neurosurgeon between 2007 and 2018 at Brigham and Women’s Hospital. Descriptive statistics and the Mantel-Cox log-rank test were used to identify differences in outcomes between these cohorts, and multivariate logistic regression was used to identify predictors of radiographic recurrence for SPAs.
Results
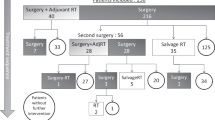
Our cohort included 88 SPAs and 200 FPAs. The majority of patients in both cohorts were female (48.9% of SPAs and 63.5% of FPAs). SPAs were larger in median diameter than FPAs (2.1 cm vs. 1.2 cm, p < 0.001). The most frequent subtypes of SPA were gonadotrophs (55.7%) and corticotrophs (30.7%). Gross total resection (GTR) was achieved in 70.1% of SPA resections and 86.0% of FPA resections (p < 0.001). SPAs had a higher likelihood of recurring (hazard ratio [HR] 3.2, 95% confidence interval [95%CI] 1.6–7.2) and a higher likelihood of requiring retreatment for recurrence (HR 2.5; 95%CI 1.0-6.1). Subset analyses revealed that recurrence and retreatment were more both likely for subtotally resected SPAs than subtotally resected FPAs, but this pattern was not observed in SPAs and FPAs after GTR. Among SPAs, recurrence was associated with STR (odds ratio [OR] 9.3; 95%CI 1.4–64.0) and younger age (OR 0.92 per year; 95%CI 0.88–0.98) in multivariable analysis. Of SPAs that recurred, 12 of 19 (63.2%) were retreated with repeat surgery (n = 11) or radiosurgery (n = 1), while the remainder were observed (n = 7).There were similar rates of recurrence across different SPA subtypes.
Conclusion
Patients undergoing resection of SPAs should be closely monitored for disease recurrence through more frequent clinical follow-up and diagnostic imaging than other adenomas, particularly among patients with STR and younger patients. Several patients can be observed after radiographic recurrence, and the decision to retreat should be individualized. Longitudinal clinical follow-up of SPAs, including an assessment of symptoms, endocrine function, and imaging remains critical.



Similar content being viewed by others
References
Famini P, Maya MM, Melmed S (2011) Pituitary magnetic resonance imaging for sellar and parasellar masses: ten-year experience in 2598 patients. J Clin Endocrinol Metab 96:1633–1641
Lake MG, Krook LS, Cruz SV (2013) Pituitary adenomas: an overview. Am Fam Physician 88:319–327
Daly AF, Beckers A (2020) The epidemiology of Pituitary Adenomas. Endocrinol Metab Clin North Am 49:347–355
Ezzat S, Asa SL, Couldwell WT et al (2004) The prevalence of pituitary adenomas: a systematic review. Cancer 101:613–619
Brochier S, Galland F, Kujas M et al (2010) Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur J Endocrinol 163:193–200
Kirkman MA, Jaunmuktane Z, Brandner S et al (2014) Active and silent thyroid-stimulating hormone-expressing pituitary adenomas: presenting symptoms, treatment, outcomes, and recurrence. World Neurosurg 82:1224–1231
Wang EL, Qian ZR, Yamada S et al (2009) Clinicopathological characterization of TSH-producing adenomas: special reference to TSH-immunoreactive but clinically non-functioning adenomas. Endocr Pathol 20:209–220
Cooper O, Melmed S (2012) Subclinical hyperfunctioning pituitary adenomas: the silent tumors. Best Pract Res Clin Endocrinol Metab 26:447
Micko A, Rötzer T, Hoftberger R et al (2020) Expression of additional transcription factors is of prognostic value for aggressive behavior of pituitary adenomas. J Neurosurg 134:1139–1146
Lee JC, Pekmezci M, Lavezo JL et al (2017) Utility of Pit-1 immunostaining in distinguishing pituitary adenomas of primitive differentiation from null cell adenomas. Endocr Pathol 28:287–292
Trouillas J, Roy P, Sturm N et al (2013) A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol 126:123–135
Wang X, Li M, Jiang X et al (2022) Prediction of higher Ki-67 index in Pituitary Adenomas by pre- and intra-operative clinical characteristics. Brain Sci 12:1002
Asa SL, Mete O, Perry A, Osamura RY (2022) Overview of the 2022 WHO classification of Pituitary tumors. Endocr Pathol 33:6–26
Lopes MBS (2017) The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol 134:521–535
Drummond J, Roncaroli F, Grossman AB, Korbonits M (2019) Clinical and pathological aspects of Silent Pituitary Adenomas. J Clin Endocrinol Metab 104:2473–2489
Molitch ME (2017) Diagnosis and treatment of Pituitary adenomas: a review. JAMA 317:516–524
Roelfsema F, Biermasz NR, Pereira AM (2012) Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary 15:71–83
Lu L, Wan X, Xu Y et al (2022) Prognostic Factors for Recurrence in Pituitary Adenomas: Recent Progress and Future Directions. Diagnostics (Basel) 12.: https://doi.org/10.3390/diagnostics12040977
Hofstetter CP, Nanaszko MJ, Mubita LL et al (2012) Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary 15:450–463
Lampropoulos KI, Samonis G, Nomikos P (2013) Factors influencing the outcome of microsurgical transsphenoidal surgery for pituitary adenomas: a study on 184 patients. Hormones 12:254–264
Ferreira JEA, de Mello PA, de Magalhães AV et al (2005) [Non-functioning pituitary adenomas: clinical features and immunohistochemistry]. Arq Neuropsiquiatr 63:1070–1078
Watts AK, Easwaran A, McNeill P et al (2017) Younger age is a risk factor for regrowth and recurrence of nonfunctioning pituitary macroadenomas: results from a single Australian centre. Clin Endocrinol 87:264–271
Richardson TE, Shen Z-J, Kanchwala M et al (2017) Aggressive behavior in Silent Subtype III Pituitary Adenomas May depend on suppression of local Immune response: a whole transcriptome analysis. J Neuropathol Exp Neurol 76:874–882
Mete O, Gomez-Hernandez K, Kucharczyk W et al (2016) Silent subtype 3 pituitary adenomas are not always silent and represent poorly differentiated monomorphous plurihormonal Pit-1 lineage adenomas. Mod Pathol 29:131–142
Funding
This study was not funded.
Author information
Authors and Affiliations
Contributions
SG and ERL contributed to project ideation. SG, SH, NM, and BH contributed to data collection. SG, SH, BH, and JDB contributed to data analysis. SG, NM, MA, TRS, OA, and ERL contributed to data interpretation. SG drafted the manuscript text. All co-authors provided critical edits. All authors reviewed the manuscript. ERL provided study supervision.
Corresponding author
Ethics declarations
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes. There are no personal identifying details of any patients in this manuscript. For retrospective de-identified clinical research, patients are given the ability to opt-out when they are admitted at our institution. To the study authors’ best knowledge, all patients approved of use of their de-identified clinical data. This study was approved under Mass General Brigham IRB Protocol #2015P002352.
Conflicts of interest
JDB has an equity position in Treovir Inc., an oHSV clinical stage company and is a member of the POCKiT Diagnostics, Centile Bioscience, and NeuroX1 Boards of Scientific Advisors. All other authors report no disclosures or conflicts of interest related to this submission.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, S., Hoffman, S.E., Mehta, N.H. et al. Elevated risk of recurrence and retreatment for silent pituitary adenomas. Pituitary 27, 204–212 (2024). https://doi.org/10.1007/s11102-024-01382-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-024-01382-3