Abstract
Purpose
Stereotactic radiosurgery (SRS) can be used in acromegaly patients to achieve endocrine remission. In this study we evaluate the biological effective dose (BED) as a predictor of SRS outcomes for acromegaly.
Method
This retrospective, single-center study included patients treated with single-fraction SRS with growth hormone secreting pituitary adenomas and available endocrine follow-up. Kaplan–Meier analysis was used to study endocrine remission, new pituitary deficit, and tumor control. Cox analyses were performed using two models [margin dose (model 2) versus BED (model 1)].
Results
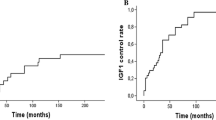
Sixty-seven patients (53.7% male) with a median age of 46.8 years (IQR 21.2) were treated using a median dose of 25 Gy (IQR 5), and a median BED of 171.9Gy2.47 (IQR 66.0). Five (7.5%) were treated without stopping antisecretory medication. The cumulative probability of maintained endocrine remission off suppressive medications was 62.5% [47.9–73.0] at 3 years and 76.5% [61.0–85.9] at 5 years. IGF1i > 1.5 was a predictor of treatment failure [Hazard ratio (HR) 0.40 (0.21–0.79) in model 1, p = 0.00783]. Margin dose > 22 Gy [HR 2.33 (1.06–5.13), p = 0.03593] or a BED > 170Gy2.47 [HR 2.02 (1.06–3.86), p = 0.03370] were associated with endocrine remission. The cumulative probability of new hypopituitarism after SRS was 36.8% (CI 95% 22.4–45.9) at 3 years and 53.2% (CI 95% 35.6–66) at 5 years. BED or margin dose were not associated with new hypopituitarism.
Conclusion
BED is a strong predictor of endocrine remission in patients treated with SRS. Dose planning and optimization of the BED to > 170Gy2.47 give a greater probability of endocrine remission in acromegalic patients.


Similar content being viewed by others
Data availability
The data can be available upon reasonable request to the corresponding author.
Abbreviations
- BED:
-
Biological effective dose
- HR:
-
Hazard ratio
- IGF1i :
-
Insulin growth factor index
- IQR:
-
Interquartile range
- Gy:
-
Gray
- OGT:
-
Oral glucose test
- SRS:
-
Stereotactic radiosurgery
References
Crisafulli S, Luxi N, Sultana J et al (2021) Global epidemiology of acromegaly: a systematic review and meta-analysis. Eur J Endocrinol 185:251–263. https://doi.org/10.1530/EJE-21-0216
Giustina A, Barkhoudarian G, Beckers A et al (2020) Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord 21:667–678. https://doi.org/10.1007/s11154-020-09588-z
Mathieu D, Kotecha R, Sahgal A et al (2022) Stereotactic radiosurgery for secretory pituitary adenomas: systematic review and International Stereotactic Radiosurgery Society practice recommendations. J Neurosurg 136:801–812. https://doi.org/10.3171/2021.2.JNS204440
Sheehan J, Lee C-C, Bodach ME et al (2016) Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery 79:E539-540. https://doi.org/10.1227/NEU.0000000000001385
Singh R, Didwania P, Lehrer EJ et al (2020) Stereotactic radiosurgery for acromegaly: an international systematic review and meta-analysis of clinical outcomes. J Neurooncol 148:401–418. https://doi.org/10.1007/s11060-020-03552-2
Ding D, Mehta GU, Patibandla MR et al (2019) Stereotactic radiosurgery for acromegaly: an international multicenter retrospective cohort study. Neurosurgery 84:717–725. https://doi.org/10.1093/neuros/nyy178
Lee C-C, Vance ML, Xu Z et al (2014) Stereotactic radiosurgery for acromegaly. J Clin Endocrinol Metab 99:1273–1281. https://doi.org/10.1210/jc.2013-3743
Liu X, Kano H, Kondziolka D et al (2012) Gamma knife radiosurgery for clinically persistent acromegaly. J Neurooncol 109:71–79. https://doi.org/10.1007/s11060-012-0862-z
Pai F-Y, Chen C-J, Wang W-H et al (2019) Low-dose gamma knife radiosurgery for acromegaly. Neurosurgery 85:E20–E30. https://doi.org/10.1093/neuros/nyy410
Wu Y, Wang M, Xu Y et al (2021) Comparing primary gamma knife radiosurgery and postoperative gamma knife radiosurgery for acromegaly: a monocenter retrospective study. Clin Neurol Neurosurg 200:106385. https://doi.org/10.1016/j.clineuro.2020.106385
Graffeo CS, Perry A, Link MJ et al (2021) Biological effective dose as a predictor of hypopituitarism after single-fraction pituitary adenoma radiosurgery: dosimetric analysis and cohort study of patients treated using contemporary techniques. Neurosurgery 88:E330–E335. https://doi.org/10.1093/neuros/nyaa555
Huo M, Rose M, van Prooijen M et al (2022) Importance of Cobalt-60 dose rate and biologically effective dose on local control for intracranial Meningiomas treated with stereotactic radiosurgery. Neurosurgery 90:140–147. https://doi.org/10.1227/NEU.0000000000001755
Tuleasca C, Peciu-Florianu I, Leroy H-A et al (2020) Biologically effective dose and prediction of obliteration of unruptured arteriovenous malformations treated by upfront gamma knife radiosurgery: a series of 149 consecutive cases. J Neurosurg. https://doi.org/10.3171/2020.4.JNS201250
Balossier A, Tuleasca C, Cortet-Rudelli C et al (2021) Gamma knife radiosurgery for acromegaly: evaluating the role of the biological effective dose associated with endocrine remission in a series of 42 consecutive cases. Clin Endocrinol (Oxford) 94:424–433. https://doi.org/10.1111/cen.14346
Graffeo CS, Donegan D, Erickson D et al (2020) The impact of insulin-like growth factor index and biologically effective dose on outcomes after stereotactic radiosurgery for acromegaly: cohort study. Neurosurgery 87:538–546. https://doi.org/10.1093/neuros/nyaa054
Sheehan JP, Pouratian N, Steiner L et al (2011) Gamma Knife surgery for pituitary adenomas: factors related to radiological and endocrine outcomes. J Neurosurg 114:303–309. https://doi.org/10.3171/2010.5.JNS091635
Melmed S, Bronstein MD, Chanson P et al (2018) A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol 14:552–561. https://doi.org/10.1038/s41574-018-0058-5
Magalhães J, Ventura N, Lamback EB et al (2022) A prospective study on the efficacy of oral estrogen in female patients with acromegaly. Pituitary 25:433–443. https://doi.org/10.1007/s11102-021-01204-w
Schilbach K, Gar C, Lechner A et al (2019) Determinants of the growth hormone nadir during oral glucose tolerance test in adults. Eur J Endocrinol 181:55–67. https://doi.org/10.1530/EJE-19-0139
Imber BS, Lin AL, Zhang Z et al (2019) Comparison of radiographic approaches to assess treatment response in pituitary adenomas: is recist or rano good enough? J Endocr Soc 3:1693–1706. https://doi.org/10.1210/js.2019-00130
Jones B, Hopewell JW (2019) Modelling the influence of treatment time on the biological effectiveness of single radiosurgery treatments: derivation of “protective” dose modification factors. Br J Radiol 92:20180111. https://doi.org/10.1259/bjr.20180111
R Core Team (2020) R: A language and environement for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Pollock BE, Jacob JT, Brown PD, Nippoldt TB (2007) Radiosurgery of growth hormone-producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg 106:833–838. https://doi.org/10.3171/jns.2007.106.5.833
Gutt B, Wowra B, Alexandrov R et al (2005) Gamma-knife surgery is effective in normalising plasma insulin-like growth factor I in patients with acromegaly. Exp Clin Endocrinol Diabetes Off J Ger Soc Endocrinol Ger Diabetes Assoc 113:219–224. https://doi.org/10.1055/s-2005-837552
Kong D-S, Kim Y-H, Kim YH et al (2019) Long-term efficacy and tolerability of gamma knife radiosurgery for growth hormone-secreting adenoma: a retrospective multicenter study (MERGE-001). World Neurosurg 122:e1291–e1299. https://doi.org/10.1016/j.wneu.2018.11.038
Losa M, Gioia L, Picozzi P et al (2008) The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J Clin Endocrinol Metab 93:2546–2552. https://doi.org/10.1210/jc.2008-0135
Hopewell JW, Millar WT, Lindquist C et al (2013) Application of the concept of biologically effective dose (BED) to patients with vestibular Schwannomas treated by radiosurgery. J Radiosurgery SBRT 2:257–271
Jones B, Klinge T, Hopewell JW (2020) The influence of the α/β ratio on treatment time iso-effect relationships in the central nervous system. Int J Radiat Biol 96:903–909. https://doi.org/10.1080/09553002.2020.1748736
Pop LA, Millar WT, van der Plas M, van der Kogel AJ (2000) Radiation tolerance of rat spinal cord to pulsed dose rate (PDR-) brachytherapy: the impact of differences in temporal dose distribution. Radiother Oncol J Eur Soc Ther Radiol Oncol 55:301–315. https://doi.org/10.1016/s0167-8140(00)00205-x
Acknowledgements
Dr Dumot gratefully acknowledges receipt of a grant for mobility from the Hospices civils de Lyon, France, from the Institut Servier, France, from the Societe française of Neurochirurgie (SFNC), France, from the Fondation Planiol, France, from the Phillip foundation.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conception and design: JPS, DS. Acquisition of data: CD, DS. Analysis and interpretation of data: CD, DS, GM. Drafting the article: CD, GM. Critically revising the article: JPS, DS, GM, SD. ZX, Reviewed submitted version of manuscript: all authors. Statistical analysis: CD, DS, GM. Study supervision: JPS.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
The center obtained review board approval for this research. Due to its restrospective nature, no written consent was necessary.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dumot, C., Schlesinger, D., Mantziaris, G. et al. Role of biological effective dose for prediction of endocrine remission in acromegaly patients treated with stereotactic radiosurgery. Pituitary 26, 124–131 (2023). https://doi.org/10.1007/s11102-022-01293-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-022-01293-1