Abstract
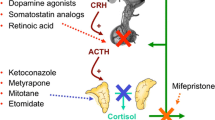
First-line treatment for Cushing´s disease is transsphenoidal surgery. But in cases of persistent or recurrent disease after surgery, contraindications to surgery, severe hypercortisolism control before surgery, or for patients waiting for radiotherapy effects, medical therapy may be indicated. Pituitary-directed agents include cabergoline and pasireotide. Both drugs present similar potential for biochemical control and pasireotide has additionally been proved to reduce tumor volume. Moreover, pasireotide was evaluated in high quality studies. In respect to safety, both drugs are well tolerated and safe, but special attention should be given for cardiac valve disease and psychiatric disorder for cabergoline, and hyperglycemia for pasireotide.
Similar content being viewed by others
References
Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, Boguszewski CL, Bronstein MD, Buchfelder M, Carmichael JD, Casanueva FF, Castinetti F, Chanson P, Findling J, Gadelha M, Geer EB, Giustina A, Grossman A, Gurnell M, Ho K, Ioachimescu AG, Kaiser UB, Karavitaki N, Katznelson L, Kelly DF, Lacroix A, McCormack A, Melmed S, Molitch M, Mortini P, Newell-Price J, Nieman L, Pereira AM, Petersenn S, Pivonello R, Raff H, Reincke M, Salvatori R, Scaroni C, Shimon I, Stratakis CA, Swearingen B, Tabarin A, Takahashi Y, Theodoropoulou M, Tsagarakis S, Valassi E, Varlamov EV, Vila G, Wass J, Webb SM, Zatelli MC, Biller (2021) B.M.K.: Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol 9, 847–875
Gatto F, Arvigo M, Ferone D (2020) Somatostatin receptor expression and patients’ response to targeted medical treatment in pituitary tumors: evidences and controversies. J Endocrinol Invest 43:1543–1553
Pivonello R, Pivonello C, Simeoli C, De Martino MC, Colao A (2022) The dopaminergic control of Cushing’s syndrome. J Endocrinol Invest. doi: https://doi.org/10.1007/s40618-021-01661-x
Ferriere A, Cortet C, Chanson P, Delemer B, Caron P, Chabre O, Reznik Y, Bertherat J, Rohmer V, Briet C, Raingeard I, Castinetti F, Beckers A, Vroonen L, Maiter D, Cephise-Velayoudom FL, Nunes ML, Haissaguerre M, Tabarin A (2017) Cabergoline for Cushing’s disease: a large retrospective multicenter study. Eur J Endocrinol 176:305–314
Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, Hofland LJ, Lamberts SW, Colao A (2009) The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 94:223–230
Godbout A, Manavela M, Danilowicz K, Beauregard H, Bruno OD, Lacroix A (2010) Cabergoline monotherapy in the long-term treatment of Cushing’s disease. Eur J Endocrinol 163:709–716
Manavela MP, Danilowicz K, Bruno OD (2012) Macrocorticotropinoma shrinkage and control of hypercortisolism under long-term cabergoline therapy: case report. Pituitary 15(Suppl 1):33–36
Casulari LA, Naves LA, Mello PA, Pereira Neto A, Papadia C (2004) Nelson’s syndrome: complete remission with cabergoline but not with bromocriptine or cyproheptadine treatment. Horm Res 62:300–305
Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BM, Group PBS (2012) A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med 366:914–924
Lacroix A, Gu F, Gallardo W, Pivonello R, Yu Y, Witek P, Boscaro M, Salvatori R, Yamada M, Tauchmanova L, Roughton M, Ravichandran S, Petersenn S, Biller BMK, Newell-Price J, Group PGS (2018) Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. The lancet. Diabetes & endocrinology 6:17–26
Lacroix A, Gu F, Schopohl J, Kandra A, Pedroncelli AM, Jin L, Pivonello R (2020) Pasireotide treatment significantly reduces tumor volume in patients with Cushing’s disease: results from a Phase 3 study. Pituitary 23:203–211
Shimon I, Rot L, Inbar E (2012) Pituitary-directed medical therapy with pasireotide for a corticotroph macroadenoma: pituitary volume reduction and literature review. Pituitary 15:608–613
Pivonello R, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Trovato A, Hughes G, Salgado LR, Lacroix A, Schopohl J, Biller BM, Group PBS (2014) Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing’s disease: results from a Phase III study. Clin Endocrinol (Oxf) 81:408–417
Lacroix A, Bronstein MD, Schopohl J, Delibasi T, Salvatori R, Li Y, Barkan A, Suzaki N, Tauchmanova L, Ortmann CE, Ravichandran S, Petersenn S, Pivonello R (2020) Long-acting pasireotide improves clinical signs and quality of life in Cushing’s disease: results from a phase III study. J Endocrinol Invest 43:1613–1622
Petersenn S, Salgado LR, Schopohl J, Portocarrero-Ortiz L, Arnaldi G, Lacroix A, Scaroni C, Ravichandran S, Kandra A, Biller BMK (2017) Long-term treatment of Cushing’s disease with pasireotide: 5-year results from an open-label extension study of a Phase III trial. Endocrine 57:156–165
Fleseriu M, Petersenn S, Biller BMK, Kadioglu P, De Block C, T’Sjoen G, Vantyghem MC, Tauchmanova L, Wojna J, Roughton M, Lacroix A, Newell-Price J (2019) Long-term efficacy and safety of once-monthly pasireotide in Cushing’s disease: A Phase III extension study. Clin Endocrinol (Oxf) 91:776–785
Hayashi K, Inoshita N, Kawaguchi K, Ibrahim Ardisasmita A, Suzuki H, Fukuhara N, Okada M, Nishioka H, Takeuchi Y, Komada M, Takeshita A, Yamada S (2016) The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur J Endocrinol 174:213–226
Steeds R, Stiles C, Sharma V, Chambers J, Lloyd G, Drake W (2019) Echocardiography and monitoring patients receiving dopamine agonist therapy for hyperprolactinaemia: A joint position statement of the British Society of Echocardiography, the British Heart Valve Society and the Society for Endocrinology. Clin Endocrinol (Oxf) 90:662–669
Hinojosa-Amaya JM, Johnson N, González-Torres C, Varlamov EV, Yedinak CG, McCartney S, Fleseriu M (2020) Depression and Impulsivity Self-Assessment Tools to Identify Dopamine Agonist Side Effects in Patients With Pituitary Adenomas. Front Endocrinol (Lausanne) 11:579606
Colao A, De Block C, Gaztambide MS, Kumar S, Seufert J, Casanueva FF (2014) Managing hyperglycemia in patients with Cushing’s disease treated with pasireotide: medical expert recommendations. Pituitary 17:180–186
Funding
The authors received no funding.
Author information
Authors and Affiliations
Contributions
MRG, LEW and IS were equally responsible for writing and reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
MRG has received speaker fees from Recordati Rare Diseases, attended Recordati Rare Diseases advisory boards, and is principal investigator in clinical trials from Recordati Rare Diseases and Novartis. LEW has received speaker fees from Novartis and participates as a sub-investigator in Recordati Rare Diseases and Novartis clinical trials. IS has received speaker fees from Novartis and Medison, attended Novartis advisory board, and participated as an investigator in Novartis and Strongbridge Pharma clinical trials.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gadelha, M.R., Wildemberg, L.E. & Shimon, I. Pituitary acting drugs: cabergoline and pasireotide. Pituitary 25, 722–725 (2022). https://doi.org/10.1007/s11102-022-01238-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-022-01238-8