Abstract
Purpose
To report the effects of pegvisomant (PEGV) treatment on patient-reported outcomes in acromegaly patients.
Methods
We conducted an extension study of an open-label, multinational, non-interventional study (ACROSTUDY) evaluating the long-term safety and efficacy of PEGV for acromegaly in routine clinical practice. Enrolled patients were rollover patients from ACROSTUDY, or treatment naïve/semi-naïve (NSN; no PEGV within 6 months of enrollment). Exploratory efficacy endpoints were changes in symptoms with the Patient-Assessed Acromegaly Symptom Questionnaire (PASQ) and quality of life with the Acromegaly Quality of Life questionnaire (AcroQoL) analyzed by controlled or uncontrolled IGF-I levels. Results were analyzed in all patients, in NSN patient subgroup, and by diabetes status.
Results
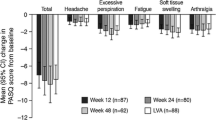
A total of 544 patients with acromegaly were enrolled, including 434 rollover subjects from ACROSTUDY and 110 NSN patients. Mean PEGV treatment duration was 7.8 years (range, 0–19.6 years). Overall, the majority of PASQ scores improved over time, but there was no significant difference between IGF-I controlled or uncontrolled groups. In the NSN subgroup, most PASQ and AcroQoL scores remained similar to baseline up to 1 year, regardless of IGF-I control. Patients with diabetes reported better PASQ scores over time with PEGV treatment, regardless of IGF-I control. IGF-I normalization increased from 10% of patients at baseline to more than 78% at year 10, with a mean daily PEGV dose of 18.7 mg.
Conclusions
Overall, patients treated with PEGV had small improvements in PASQ. While IGF-I normalization increased with PEGV treatment, IGF-I control had no effects on PASQ and AcroQoL scores.




Similar content being viewed by others
References
Melmed S (2006) Medical progress: acromegaly. N Engl J Med 355(24):2558–2573
Akirov A, Asa SL, Amer L, Shimon I, Ezzat S (2019) The clinicopathological spectrum of acromegaly. J Clin Med 8(11):1962
Vilar L, Vilar CF, Lyra R, Lyra R, Naves LA (2017) Acromegaly: clinical features at diagnosis. Pituitary 20(1):22–32
Melmed S (2009) Acromegaly pathogenesis and treatment. J Clin Invest 119(11):3189–3202
Maione L, Brue T, Beckers A, Delemer B, Petrossians P, Borson-Chazot F, Chabre O, Francois P, Bertherat J, Cortet-Rudelli C et al (2017) Changes in the management and comorbidities of acromegaly over three decades: the French Acromegaly Registry. Eur J Endocrinol 176(5):645–655
Gadelha MR, Kasuki L, Lim DST, Fleseriu M (2019) Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev 40(1):268–332
Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, Bolanowski M, Bonert V, Bronstein MD, Casanueva FF et al (2019) A consensus on the diagnosis and treatment of acromegaly comorbidities: an update. J Clin Endocrinol Metab 105(4):dgz096
Fleseriu M, Biller BMK, Freda PU, Gadelha MR, Giustina A, Katznelson L, Molitch ME, Samson SL, Strasburger CJ, van der Lely AJ et al (2021) A Pituitary Society update to acromegaly management guidelines. Pituitary 24(1):1–13
Strasburger CJ, Karavitaki N, Stormann S, Trainer PJ, Kreitschmann-Andermahr I, Droste M, Korbonits M, Feldmann B, Zopf K, Sanderson VF et al (2016) Patient-reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly. Eur J Endocrinol 174(3):355–362
Geer EB, Sisco J, Adelman DT, Ludlam WH, Haviv A, Liu S, Mathias SD, Gelbaum D, Shi L (2020) Patient reported outcome data from acromegaly patients treated with injectable somatostatin receptor ligands (SRLs) in routine clinical practice. BMC Endocr Disord 20(1):117
Fleseriu M, Molitch M, Dreval A, Biermasz NR, Gordon MB, Crosby RD, Ludlam WH, Haviv A, Gilgun-Sherki Y, Mathias SD (2021) Disease and treatment-related burden in patients with acromegaly who are biochemically controlled on injectable somatostatin receptor ligands. Front Endocrinol (Lausanne) 12:627711
Fleseriu M, Fogelfeld L, Gordon MB, Sisco J, Crosby RD, Ludlam WH, Haviv A, Mathias SD (2020) An evaluation of the Acromegaly Treatment Satisfaction Questionnaire (Acro-TSQ) in adult patients with acromegaly, including correlations with other patient-reported outcome measures: data from two large multicenter international studies. Pituitary 23(4):347–358
Buchfelder M, van der Lely AJ, Biller BMK, Webb SM, Brue T, Strasburger CJ, Ghigo E, Camacho-Hubner C, Pan K, Lavenberg J et al (2018) Long-term treatment with pegvisomant: observations from 2090 acromegaly patients in ACROSTUDY. Eur J Endocrinol 179(6):419–427
Hayes LD, Sculthorpe N, Herbert P, Baker JS, Spagna R, Grace FM (2015) Six weeks of conditioning exercise increases total, but not free testosterone in lifelong sedentary aging men.Aging Male:1–6
SOMAVERT (pegvisomant (for injection, for subcutaneous use Prescribing Information. Pfizer. New York, NY. 2013)
Melmed S, Bronstein MD, Chanson P, Klibanski A, Casanueva FF, Wass JAH, Strasburger CJ, Luger A, Clemmons DR, Giustina A (2018) A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol 14(9):552–561
Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, Bolanowski M, Bonert V, Bronstein MD, Casanueva FF et al (2020) A consensus on the diagnosis and treatment of acromegaly comorbidities: an update.J Clin Endocrinol Metab105(4)
van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L, Klibanski A, Herman-Bonert V, Melmed S, Vance ML et al (2001) Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet 358(9295):1754–1759
Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ, Dimaraki EV, Stewart PM, Friend KE, Vance ML et al (2000) Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med 342(16):1171–1177
Badia X, Webb SM, Prieto L, Lara N (2004) Acromegaly Quality of Life Questionnaire (AcroQoL). Health Qual Life Outcomes 2:13
Webb SM, Prieto L, Badia X, Albareda M, Catala M, Gaztambide S, Lucas T, Paramo C, Pico A, Lucas A et al (2002) Acromegaly Quality of Life Questionnaire (ACROQOL) a new health-related quality of life questionnaire for patients with acromegaly: development and psychometric properties. Clin Endocrinol (Oxf) 57(2):251–258
Schreiber I, Buchfelder M, Droste M, Forssmann K, Mann K, Saller B, Strasburger CJ, German Pegvisomant I (2007) Treatment of acromegaly with the GH receptor antagonist pegvisomant in clinical practice: safety and efficacy evaluation from the German Pegvisomant Observational Study. Eur J Endocrinol 156(1):75–82
Sievers C, Brübach K, Saller B, Schneider HJ, Buchfelder M, Droste M, Mann K, Strasburger CJ, Stalla GK (2010) Change of symptoms and perceived health in acromegalic patients on pegvisomant therapy: a retrospective cohort study within the German Pegvisomant Observational Study (GPOS). Clin Endocrinol (Oxf) 73(1):89–94
van der Lely AJ, Gomez R, Pleil A, Badia X, Brue T, Buchfelder M, Burman P, Clemmons D, Ghigo E, Jorgensen JOL et al (2017) Development of ACRODAT((R)), a new software medical device to assess disease activity in patients with acromegaly. Pituitary 20(6):692–701
Feola T, Cozzolino A, Simonelli I, Sbardella E, Pozza C, Giannetta E, Gianfrilli D, Pasqualetti P, Lenzi A, Isidori AM (2019) Pegvisomant improves glucose metabolism in acromegaly: a meta-analysis of prospective interventional studies. J Clin Endocrinol Metab 104(7):2892–2902
Brue T, Lindberg A, van der Jan A, Akerblad AC, Koltowska-Haggstrom M, Gomez R, Droste M, Hey-Hadavi J, Strasburger CJ, Camacho-Hubner C (2019) Diabetes in patients with acromegaly treated with pegvisomant: observations from acrostudy. Endocrine 63(3):563–572
Biermasz NR, van Thiel SW, Pereira AM, Hoftijzer HC, van Hemert AM, Smit JW, Romijn JA, Roelfsema F (2004) Decreased quality of life in patients with acromegaly despite long-term cure of growth hormone excess. J Clin Endocrinol Metab 89(11):5369–5376
Broersen LHA, Zamanipoor Najafabadi AH, Pereira AM, Dekkers OM, van Furth WR, Biermasz NR (2021) Improvement in symptoms and health-related quality of life in acromegaly patients: a systematic review and meta-analysis. J Clin Endocrinol Metab 106(2):577–587
Webb SM (2011) Pituitary tumors: coping with ‘cured’ pituitary tumors. Nat Rev Endocrinol 7(5):251–252
Ben-Shlomo A, Sheppard MC, Stephens JM, Pulgar S, Melmed S (2011) Clinical, quality of life, and economic value of acromegaly disease control. Pituitary 14(3):284–294
Kyriakakis N, Lynch J, Gilbey SG, Webb SM, Murray RD (2017) Impaired quality of life in patients with treated acromegaly despite long-term biochemically stable disease: results from a 5-years prospective study. Clin Endocrinol (Oxf) 86(6):806–815
Tiemensma J, Kaptein AA, Pereira AM, Smit JW, Romijn JA, Biermasz NR (2011) Affected illness perceptions and the association with impaired quality of life in patients with long-term remission of acromegaly. J Clin Endocrinol Metab 96(11):3550–3558
Geraedts VJ, Andela CD, Stalla GK, Pereira AM, van Furth WR, Sievers C, Biermasz NR (2017) Predictors of quality of life in acromegaly: no consensus on biochemical parameters. Front Endocrinol (Lausanne) 8:40
Andela CD, Biermasz NR, Kaptein AA, Pereira AM, Tiemensma J (2015) More concerns and stronger beliefs about the necessity of medication in patients with acromegaly are associated with negative illness perceptions and impairment in quality of life. Growth Horm IGF Res 25(5):219–226
Crespo I, Valassi E, Webb SM (2017) Update on quality of life in patients with acromegaly. Pituitary 20(1):185–188
Yoshida K, Fukuoka H, Matsumoto R, Bando H, Suda K, Nishizawa H, Iguchi G, Ogawa W, Webb SM, Takahashi Y (2015) The quality of life in acromegalic patients with biochemical remission by surgery alone is superior to that in those with pharmaceutical therapy without radiotherapy, using the newly developed Japanese version of the AcroQoL. Pituitary 18(6):876–883
Webb SM, Badia X (2016) Quality of life in acromegaly. Neuroendocrinology 103(1):106–111
Caron PJ, Bevan JS, Petersenn S, Houchard A, Sert C, Webb SM, Group PI (2016) Effects of lanreotide autogel primary therapy on symptoms and quality-of-life in acromegaly: data from the PRIMARYS study. Pituitary 19(2):149–157
Brue T, Chanson P, Rodien P, Delemer B, Drui D, Marie L, Juban L, Salvi L, Henocque R, Raverot G (2021) Cost-Utility of Acromegaly Pharmacological Treatments in a French Context. Front Endocrinol (Lausanne) 12:745843
Sardella C, Lombardi M, Rossi G, Cosci C, Brogioni S, Scattina I, Webb SM, Gasperi M, Martino E, Bogazzi F (2010) Short- and long-term changes of quality of life in patients with acromegaly: results from a prospective study. J Endocrinol Invest 33(1):20–25
Webb SM, Badia X, Surinach NL, Spanish AcroQol Study G (2006) Validity and clinical applicability of the acromegaly quality of life questionnaire, AcroQoL: a 6-month prospective study. Eur J Endocrinol 155(2):269–277
Badia X, Trainer P, Biermasz NR, Tiemensma J, Carreno A, Roset M, Forsythe A, Webb SM (2018) Mapping AcroQoL scores to EQ-5D to obtain utility values for patients with acromegaly. J Med Econ 21(4):382–389
Sibeoni J, Manolios E, Verneuil L, Chanson P, Revah-Levy A (2019) Patients’ perspectives on acromegaly diagnostic delay: a qualitative study. Eur J Endocrinol 180(6):339–352
Fleseriu M, Fogelfeld L, Gordon MB, Sisco J, Colwell HH, Ludlam WH, Haviv A, Mathias SD (2019) Development of a novel patient-reported measure for acromegaly: the Acro-TSQ. Pituitary 22(6):581–593
Neggers SJ, van Aken MO, de Herder WW, Feelders RA, Janssen JA, Badia X, Webb SM, van der Lely AJ (2008) Quality of life in acromegalic patients during long-term somatostatin analog treatment with and without pegvisomant. J Clin Endocrinol Metab 93(10):3853–3859
Acknowledgements
The authors would like to thank all investigators, sub-investigators and study coordinators and patients for their participation and contributions to ACROSTUDY. This study was sponsored by Pfizer Inc. The authors also want to acknowledge the medical writing assistance of Hui Zhang, Ph.D., and Dominique Verlaan, Ph.D., CMPP of Precise Publications, LLC, which was supported by Pfizer Inc.
Funding
This study was sponsored by Pfizer. Editorial/medical writing support was provided by Hui Zhang, Ph.D., and Dominique Verlaan, Ph.D., CMPP at Precise Publications, LLC and was funded by Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RS is an editor for Pituitary; MF and TB are on the Editorial Board of Pituitary. RS is a principal investigator with research support to Johns Hopkins University for clinical research studies with Crinetics, Novartis, and Chiasma and occasional scientific consultant for Ipsen and Recordati. PM has received honoraria as consultant/speaker, and is a principal investigator for research grants from CamurusAB, Ipsen, Novartis, Pfizer. SMW has received honoraria as consultant/speaker or is a principal investigator for research grants from: Pfizer, Novartis, Ipsen, Recordati, HRA, Crinetics and Corcept. TB has received honoraria as consultant/speaker, or is a principal investigator for research grants from: Pfizer, Novartis Pharma, Ipsen Pharma, Recordati, Merck-Serono, Sandoz, Novo-Nordisk, Advanz Pharma, and Corcept. JL, MPW, SRV, and RG are employees of Pfizer and are stockholders of Pfizer. MF is a principal investigator with research support at Oregon Health & Science University for clinical research studies with Crinetics, Novartis, Recordati, Chiasma, Ionis and occasional scientific consultant for Crinetics, Pfizer, Ipsen, Recordati, and Chiasma.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salvatori, R., Maffei, P., Webb, S.M. et al. Patient-reported outcomes in patients with acromegaly treated with pegvisomant in the ACROSTUDY extension: A real-world experience. Pituitary 25, 420–432 (2022). https://doi.org/10.1007/s11102-022-01206-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-022-01206-2