Abstract
Purpose
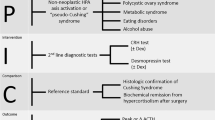
To develop an index for the differential diagnosis of corticotropin-dependent Cushing syndrome (CS).
Methods
The development cohort included 112 consecutive patients with clinicopathologically confirmed corticotropin-dependent CS at the Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital, from December 2004 to May 2020, and data of 126 patients from studies published from 2016 to August 2020, identified through search in PubMed, Embase and the Cochrane Library, was extracted for external validation. The index was calculated as the product of plasma adrenocorticotropic hormone (ACTH, pmol/L) and urinary free cortisol (UFC, nmol/24 h) divided by 10,000. The discriminative ability was tested using receiver operating characteristics (ROC) curve analysis.
Results
In development cohort, area under curve of ROC analysis of the ACTH-UFC index in identifying Cushing disease (CD) was 0.977. The diagnostic accuracy of ACTH-UFC index ≤ 11 was comparable to that of 48 h 8 mg/d high-dose dexamethasone test (HDDST) in identifying CD, with sensitivity, specificity, positive and negative likelihood ratios of 96.6%, 87.5%, 7.73, and 0.04, respectively. The sensitivity of ACTH-UFC index ≤ 11 in parallel combination with pituitary magnetic resonance imaging (MRI) was 100% for identifying CD. The performance of the ACTH-UFC index in parallel or serial combination with pituitary MRI was similar in the validation cohort.
Conclusions
ACTH-UFC index provides a rapid, convenient and non-invasive adjunctive approach for the differential diagnosis of corticotropin-dependent CS, with no risk of aggravating metabolic disturbances. Investigations for ectopic causes of corticotropin-dependent CS should be performed with ACTH-UFC index > 11 and negative contrasted pituitary MRI.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Code availability
Analytical code of the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Newell-Price J, Bertagna X, Grossman AB, Nieman LK (2006) Cushing’s syndrome. Lancet 367 (9522):1605–1617. https://doi.org/10.1016/s0140-6736(06)68699-6
Newell-Price J, Grossman AB (2001) The differential diagnosis of Cushing’s syndrome. Ann Endocrinol 62 (2):173–179
Aron DC, Raff H, Findling JW (1997) Effectiveness versus efficacy: the limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab 82 (6):1780–1785. https://doi.org/10.1210/jcem.82.6.3991
Zampetti B, Grossrubatscher E, Dalino Ciaramella P, Boccardi E, Loli P (2016) Bilateral inferior petrosal sinus sampling. Endocr Connect 5 (4):R12–R25. https://doi.org/10.1530/EC-16-0029
Loriaux DL (2017) Diagnosis and differential diagnosis of Cushing’s Syndrome. N Engl J Med 376 (15):1451–1459. https://doi.org/10.1056/NEJMra1505550
Gilbert R, Lim EM (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. Clin Biochem Rev 29 (3):103–106
Findling JW, Raff H, Aron DC (2004) The low-dose dexamethasone suppression test: a reevaluation in patients with Cushing’s Syndrome. J Clin Endocrinol Metab 89 (3):1222–1226. https://doi.org/10.1210/jc.2003-030207
Juszczak A, Grossman A (2013) The management of Cushing’s disease-from investigation to treatment. Endokrynol Polska 64 (2):166–174
Lim CT, Khoo B (2020) Normal physiology of ACTH and GH release in the hypothalamus and anterior pituitary in man. Endotext [Internet]
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, Kressel HY, Rifai N, Golub RM, Altman DG, Hooft L, Korevaar DA, Cohen JF (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 351:h5527. https://doi.org/10.1136/bmj.h5527
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28 (7):412–419. https://doi.org/10.1007/bf00280883
Yanovski JA, Cutler GB Jr, Chrousos GP, Nieman LK (1998) The dexamethasone-suppressed corticotropin-releasing hormone stimulation test differentiates mild Cushing’s disease from normal physiology. J Clin Endocrinol Metab 83 (2):348–352. https://doi.org/10.1210/jcem.83.2.4568
Isidori AM, Lenzi A (2007) Ectopic ACTH syndrome. Arq Bras Endocrinol Metabol 51 (8):1217–1225. https://doi.org/10.1590/s0004-27302007000800007
Raff H, Sharma ST, Nieman LK (2014) Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol 4 (2):739–769. https://doi.org/10.1002/cphy.c130035
Monaghan PJ, Kyriacou A, Sturgeon C, Davies A, Trainer PJ, White A, Higham CE (2016) Proopiomelanocortin interference in the measurement of adrenocorticotrophic hormone: a United Kingdom National External Quality Assessment Service study. Clin Endocrinol 85 (4):569–574. https://doi.org/10.1111/cen.13118
Page-Wilson G, Freda PU, Jacobs TP, Khandji AG, Bruce JN, Foo ST, Meece K, White A, Wardlaw SL (2014) Clinical utility of plasma POMC and AgRP measurements in the differential diagnosis of ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab 99 (10):E1838–E1845. https://doi.org/10.1210/jc.2014-1448
Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F (1999) Diagnosis and management of Cushing’s syndrome: results of an Italian multicentre study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J Clin Endocrinol Metab 84 (2):440–448. https://doi.org/10.1210/jcem.84.2.5465
Valassi E, Santos A, Yaneva M, Tóth M, Strasburger CJ, Chanson P, Wass JA, Chabre O, Pfeifer M, Feelders RA, Tsagarakis S, Trainer PJ, Franz H, Zopf K, Zacharieva S, Lamberts SW, Tabarin A, Webb SM (2011) The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol 165 (3):383–392. https://doi.org/10.1530/eje-11-0272
Blunt SB, Sandler LM, Burrin JM, Joplin GF (1990) An evaluation of the distinction of ectopic and pituitary ACTH dependent Cushing’s syndrome by clinical features, biochemical tests and radiological findings. Q J Med 77 (283):1113–1133. https://doi.org/10.1093/qjmed/77.2.1113
Chong BW, Kucharczyk W, Singer W, George S (1994) Pituitary gland MR: a comparative study of healthy volunteers and patients with microadenomas. Am J Neuroradiol 15 (4):675–679
Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH (1994) Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 120 (10):817–820. https://doi.org/10.7326/0003-4819-120-10-199405150-00001
Zemskova MS, Gundabolu B, Sinaii N, Chen CC, Carrasquillo JA, Whatley M, Chowdhury I, Gharib AM, Nieman LK (2010) Utility of various functional and anatomic imaging modalities for detection of ectopic adrenocorticotropin-secreting tumors. J Clin Endocrinol Metab 95 (3):1207–1219. https://doi.org/10.1210/jc.2009-2282
Swearingen B, Katznelson L, Miller K, Grinspoon S, Waltman A, Dorer DJ, Klibanski A, Biller BM (2004) Diagnostic errors after inferior petrosal sinus sampling. J Clin Endocrinol Metab 89 (8):3752–3763. https://doi.org/10.1210/jc.2003-032249
Chen S, Chen K, Wang S, Zhu H, Lu L, Zhang X, Tong A, Pan H, Wang R, Lu Z (2020) The optimal cut-off of BIPSS in differential diagnosis of ACTH-dependent Cushing’s syndrome: is stimulation necessary? J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz194
Yogi-Morren D, Habra MA, Faiman C, Bena J, Hatipoglu B, Kennedy L, Weil RJ, Hamrahian AH (2015) Pituitary MRI findings in patients with pituitary and ectopic ACTH-dependent Cushing syndrome: Does a 6-mm pituitary tumor size cut-off value exclude ectopic ACTH syndrome? Endocr Pract 21 (10):1098–1103. https://doi.org/10.4158/EP15662.OR
Lacroix A, Feelders RA, Stratakis CA, Nieman LK (2015) Cushing’s syndrome. Lancet 386 (9996):913–927. https://doi.org/10.1016/S0140-6736(14)61375-1
Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A (2015) Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 100 (8):2807–2831. https://doi.org/10.1210/jc.2015-1818
Frete C, Corcuff JB, Kuhn E, Salenave S, Gaye D, Young J, Chanson P, Tabarin A (2020) Non-invasive diagnostic strategy in ACTH-dependent Cushing’s Syndrome. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgaa409
Taylor RL, Machacek D, Singh RJ (2002) Validation of a high-throughput liquid chromatography–tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem 48 (9):1511–1519. https://doi.org/10.1093/clinchem/48.9.1511
Acknowledgements
We thank Dr. Junping Wang for assistance in key issues regarding radiology.
Funding
We acknowledge the supports of the National Key R&D Program of China 2019YFA0802502, the National Natural Science Foundation of China 81830025 and 81620108004, and the Tianjin Municipal Science and Technology Commission 17ZXMFSY00150, and 19JCQNJC11800.
Author information
Authors and Affiliations
Contributions
ML is the guarantor, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the study conception and design, material preparation, and data collection. Data analysis were performed by LD, BW, TC, PL, WG, YF. The first draft of the manuscript was written by Li Ding, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have nothing to disclose.
Ethical approval
The study was approved by the institutional review board, and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent of the present study patients was not obtained because the study was a secondary data analysis which not including personally identified information.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Li Ding, Baoping Wang and Tingting Chen contributed equally to this work.
Supplementary Information
Below is the link to the Supplementary Information.
Rights and permissions
About this article
Cite this article
Ding, L., Wang, B., Chen, T. et al. Development and validation of a novel index for the differential diagnosis of corticotropin‐dependent Cushing syndrome. Pituitary 24, 507–516 (2021). https://doi.org/10.1007/s11102-021-01126-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-021-01126-7