Abstract
Context
Somatostatin (SST) and dopamine (DA) inhibit growth hormone (GH) secretion and proliferation of GH-secreting pituitary adenomas (GHomas) through binding to SSTR2 and D2R receptors. Chimeric SST-DA compounds (Dopastatins) display increased potency in inhibiting GH secretion, as compared with individual SST or DA analogs (alone or combined).
Objective
To assess the efficacy of a second-generation dopastatin, TBR-065, in suppressing GH secretion from human GH- and GH/prolactin(PRL)-omas.
Design
We compared the ability of TBR-065 to inhibit GH secretion from primary cultures of human GH- or GH/PRLoma cells to that of the first generation dopastatin, TBR-760 (formerly BIM-23A760), octreotide (OCT) and cabergoline (CAB), the later either alone or combined. We investigated whether there was any impact of BIM-133, the metabolite of TBR-065, on the ability of TBR-065 to inhibit GH in these cultures.
Methods
17 GH- and GH/PRLomas were included in this study. Inhibition of GH secretion by TBR-065, TBR-760, OCT and CAB (0.1 pM to 0.1 µM) was assessed over a period of 8 h.
Results
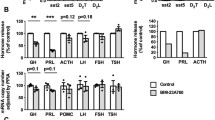
All tumors expressed SSTR2 and D2R mRNAs. GH suppression was higher with TBR-065 as compared with TBR-760 (Emax = 57 ± 5.6% vs. 41.1 ± 12.5%, respectively, p < 0.001) or with OCT + CAB (Emax = 56.8 ± 7.2% vs. 44.4 ± 9.4%, p < 0.001). BIM-133 did not have any impact on the activity of TBR-065.
Conclusion
TBR-065 has significantly improved efficacy in suppressing GH secretion as compared to current available therapies and may represent a new promising option for the treatment of acromegaly.




Similar content being viewed by others
References
Colao A, Grasso LFS, Giustina A et al (2019) Acromegaly. Nat Rev Dis Prim 5:20. https://doi.org/10.1038/s41572-019-0071-6
Gadelha MR, Kasuki L, Lim DST, Fleseriu M (2019) Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev 40:268–332. https://doi.org/10.1210/er.2018-00115
Giustina A, Barkhoudarian G, Beckers A et al (2020) Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord 21:667–678. https://doi.org/10.1007/s11154-020-09588-z
Fleseriu M, Biller BMK, Freda PU et al (2020) A Pituitary Society update to acromegaly management guidelines. Pituitary. https://doi.org/10.1007/s11102-020-01091-7
Maione L, Chanson P (2019) National acromegaly registries. Best Pract Res Clin Endocrinol Metab 33:101264. https://doi.org/10.1016/j.beem.2019.02.001
Gatto F, Feelders RA, van der Pas R et al (2013) Immunoreactivity score using an anti-sst2A receptor monoclonal antibody strongly predicts the biochemical response to adjuvant treatment with somatostatin analogs in acromegaly. J Clin Endocrinol Metab 98:E66–E71. https://doi.org/10.1210/jc.2012-2609
Chinezu L, Vasiljevic A, Jouanneau E et al (2014) Expression of somatostatin receptors, SSTR2A and SSTR5, in 108 endocrine pituitary tumors using immunohistochemical detection with new specific monoclonal antibodies. Hum Pathol 45:71–77. https://doi.org/10.1016/j.humpath.2013.08.007
Casar-Borota O, Heck A, Schulz S et al (2013) Expression of SSTR2a, but not of SSTRs 1, 3, or 5 in somatotroph adenomas assessed by monoclonal antibodies was reduced by octreotide and correlated with the acute and long-term effects of octreotide. J Clin Endocrinol Metab 98:E1730–E1739. https://doi.org/10.1210/jc.2013-2145
Hofland LJ, Feelders RA, de Herder WW, Lamberts SWJ (2010) Pituitary tumours: the sst/D2 receptors as molecular targets. Mol Cell Endocrinol 326:89–98. https://doi.org/10.1016/j.mce.2010.04.020
Saveanu A, Jaquet P, Brue T, Barlier A (2008) Relevance of coexpression of somatostatin and dopamine D2 receptors in pituitary adenomas. Mol Cell Endocrinol 286:206–213. https://doi.org/10.1016/j.mce.2007.12.008
Rocheville M, Lange DC, Kumar U et al (2000) Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J Biol Chem 275:7862–7869. https://doi.org/10.1074/jbc.275.11.7862
Gatto F, Hofland LJ (2011) The role of somatostatin and dopamine D2 receptors in endocrine tumors. Endocr Relat Cancer 18:R233–R251. https://doi.org/10.1530/ERC-10-0334
Durán-Prado M, Malagón MM, Gracia-Navarro F, Castaño JP (2008) Dimerization of G protein-coupled receptors: new avenues for somatostatin receptor signalling, control and functioning. Mol Cell Endocrinol 286:63–68. https://doi.org/10.1016/j.mce.2007.12.006
Baragli A, Alturaihi H, Watt HL et al (2007) Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2. Cell Signal 19:2304–2316. https://doi.org/10.1016/j.cellsig.2007.07.007
Hofland LJ, Lamberts SWJ (2003) The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 24:28–47. https://doi.org/10.1210/er.2000-0001
Saveanu A, Lavaque E, Gunz G et al (2002) Demonstration of enhanced potency of a chimeric somatostatin-dopamine molecule, BIM-23A387, in suppressing growth hormone and prolactin secretion from human pituitary somatotroph adenoma cells. J Clin Endocrinol Metab 87:5545–5552. https://doi.org/10.1210/jc.2002-020934
Ren S-G, Kim S, Taylor J et al (2003) Suppression of rat and human growth hormone and prolactin secretion by a novel somatostatin/dopaminergic chimeric ligand. J Clin Endocrinol Metab 88:5414–5421. https://doi.org/10.1210/jc.2003-030302
Gruszka A, Culler MD, Melmed S (2012) Somatostatin analogs and chimeric somatostatin-dopamine molecules differentially regulate human growth hormone and prolactin gene expression and secretion in vitro. Mol Cell Endocrinol 362:104–109. https://doi.org/10.1016/j.mce.2012.05.020
Culler M (2011) Somatostatin-dopamine chimeras: a novel approach to treatment of neuroendocrine tumors. Horm Metab Res 43:854–857. https://doi.org/10.1055/s-0031-1287769
Hill J, Kim S, Tsomaia N et al (2017) Chimeric somatostatin-dopamine compounds (dopastatins) for the treatment of neuroendocrine disease. In: Comprehensive medicinal chemistry III. Elsevier, Amsterdam
Jaquet P, Ouafik L, Saveanu A et al (1999) Quantitative and functional expression of somatostatin receptor subtypes in human prolactinomas. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.84.9.3268
Jaquet P, Gunz G, Grisoli F (1985) Hormonal regulation of prolactin release by human prolactinoma cells cultured in serum-free conditions. Horm Res 22:153–163
Castinetti F, Régis J, Dufour H, Brue T (2010) Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat Rev Endocrinol 6:214–223. https://doi.org/10.1038/nrendo.2010.4
Knappe UJ, Petroff D, Quinkler M et al (2020) Fractionated radiotherapy and radiosurgery in acromegaly: analysis of 352 patients from the German Acromegaly Registry. Eur J Endocrinol 182:275–284. https://doi.org/10.1530/EJE-19-0784
van der Lely AJ, Hutson RK, Trainer PJ et al (2001) Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet (London, England) 358:1754–1759. https://doi.org/10.1016/s0140-6736(01)06844-1
Gadelha MR, Bronstein MD, Brue T et al (2014) Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol 2:875–884. https://doi.org/10.1016/S2213-8587(14)70169-X
Coopmans EC, Muhammad A, van der Lely AJ et al (2019) How to position pasireotide LAR treatment in acromegaly. J Clin Endocrinol Metab 104:1978–1988. https://doi.org/10.1210/jc.2018-01979
Bruns C, Lewis I, Briner U et al (2002) SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 146:707–716. https://doi.org/10.1530/eje.0.1460707
Jaquet P, Gunz G, Saveanu A et al (2005) Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. Eur J Endocrinol 153:135–141. https://doi.org/10.1530/eje.1.01950
Florio T, Barbieri F, Spaziante R et al (2008) Efficacy of a dopamine-somatostatin chimeric molecule, BIM-23A760, in the control of cell growth from primary cultures of human non-functioning pituitary adenomas: a multi-center study. Endocr Relat Cancer 15:583–596. https://doi.org/10.1677/ERC-07-0271
Gatto F, Barbieri F, Gatti M et al (2012) Balance between somatostatin and D2 receptor expression drives TSH-secreting adenoma response to somatostatin analogues and dopastatins. Clin Endocrinol (Oxf) 76:407–414. https://doi.org/10.1111/j.1365-2265.2011.04200.x
Vázquez-Borrego MC, L-López F, Gálvez-Moreno MA et al (2020) A new generation somatostatin-dopamine analogue exerts potent antitumoral actions on pituitary neuroendocrine tumor cells. Neuroendocrinology 110:70–82. https://doi.org/10.1159/000500812
De Boon WMI, Van Esdonk MJ, Stuurman FE et al (2018) A novel somatostatin-dopamine chimera (BIM23B065) reduced GH secretion in a first-in-human clinical trial. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2018-01364
Couvelard A, Pélaprat D, Dokmak S et al (2017) Antisecretory effects of chimeric somatostatin/dopamine receptor ligands on gastroenteropancreatic neuroendocrine tumors. Pancreas 46:631–638. https://doi.org/10.1097/MPA.0000000000000813
Günther T, Culler M, Schulz S (2016) Research resource: real-time analysis of somatostatin and dopamine receptor signaling in pituitary cells using a fluorescence-based membrane potential assay. Mol Endocrinol 30:479–490. https://doi.org/10.1210/me.2015-1241
Gatto F, Feelders RA, Franck SE et al (2017) In vitro head-to-head comparison between octreotide and pasireotide in GH-secreting pituitary adenomas. J Clin Endocrinol Metab 102:2009–2018. https://doi.org/10.1210/jc.2017-00135
Ibáñez-Costa A, López-Sánchez LM, Gahete MD et al (2017) BIM-23A760 influences key functional endpoints in pituitary adenomas and normal pituitaries: molecular mechanisms underlying the differential response in adenomas. Sci Rep 7:42002. https://doi.org/10.1038/srep42002
Dicitore A, Cantone MC, Gaudenzi G et al (2020) Efficacy of a novel second-generation somatostatin-dopamine chimera (TBR-065) in human medullary thyroid cancer: a preclinical study. Neuroendocrinology. https://doi.org/10.1159/000512366
Kim J, Oh JH, Harlem H et al (2020) Therapeutic effect of a novel chimeric molecule targeting both somatostatin and dopamine receptors on growth hormone-secreting pituitary adenomas. Endocrinol Metab (Seoul, Korea) 35:177–187. https://doi.org/10.3803/EnM.2020.35.1.177
Herrera-Martínez AD, van den Dungen R, Dogan-Oruc F et al (2019) Effects of novel somatostatin-dopamine chimeric drugs in 2D and 3D cell culture models of neuroendocrine tumors. Endocr Relat Cancer 26:585–599. https://doi.org/10.1530/ERC-19-0086
Funding
This work was supported by IPSEN research grant, Centre National de la Recherche Scientifique (CNRS UMR 7286), Institut National de la Santé et de la Recherche Médicale (INSERM, UMR 1251) Aix Marseille University, Association pour le Developpement et la Recherche Médicales (ADEREM). The project leading to this publication has received funding from "Excellence Initiative of Aix Marseille University A*Midex-a French "Investissement d'Avenir".
Author information
Authors and Affiliations
Contributions
All authors contributed to the redaction of the manuscript. TC and AS reviewed it. All of the coauthors approved it.
Corresponding author
Ethics declarations
Conflict of interest
RD, SZ, TL, HH, MC have competing interest related to this study. None of the others authors have conflict of interest related to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cuny, T., Graillon, T., Defilles, C. et al. Characterization of the ability of a, second-generation SST-DA chimeric molecule, TBR-065, to suppress GH secretion from human GH-secreting adenoma cells. Pituitary 24, 351–358 (2021). https://doi.org/10.1007/s11102-020-01113-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-020-01113-4