Abstract
Purpose
To investigate whether radiomic features from magnetic resonance image (MRI) can predict the granulation pattern of growth hormone (GH)-secreting pituitary adenoma patients.
Methods
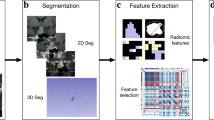
Sixty-nine pathologically proven acromegaly patients (densely granulated [DG] = 50, sparsely granulated [SG] = 19) were included. Radiomic features (n = 214) were extracted from contrast-enhancing and total tumor portions from T2-weighted (T2) MRIs. Imaging features were selected using a least absolute shrinkage and selection operator (LASSO) logistic regression model with fivefold cross-validation. Diagnostic performance for predicting granulation pattern was compared with that for qualitative T2 signal intensity assessment and T2 relative signal intensity (rSI) using the area under the receiver operating characteristics curve (AUC).
Results
Four significant radiomic features from the contrast-enhancing tumor (1 from shape, 1 from first order feature, and 2 from second order features) were selected by LASSO for model construction. The radiomics model showed an AUC, accuracy, sensitivity, and specificity of 0.834 (95% confidence interval [CI] 0.738–0.930), 73.7%, 74.0%, and 73.9%, respectively. The radiomics model showed significantly better performance than the model using qualitative T2 signal intensity assessment (AUC 0.597 [95% CI 0.447–0.747], P = 0.009) and T2 rSI (AUC 0.647 [95% CI 0.523–0.759], P = 0.037).
Conclusion
Radiomic features may be useful biomarkers to differentiate granulation pattern of GH-secreting pituitary adenoma patients, and showed better performance than qualitative assessment or rSI evaluation.



Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are not publicly available due its proprietary nature, but are available from the corresponding author for validation of our results upon request with permission from all of the co-authors.
Code availability
The code for statistical analysis performed by statistical software R (version 4.0.1; R Foundation for Statistical Computing, Vienna, Austria) is available from the corresponding author for validation of our results upon request with permission from all of the co-authors.
References
Melmed S (2009) Acromegaly pathogenesis and treatment. J Clin Invest 119(11):3189–3202. https://doi.org/10.1172/jci39375
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951. https://doi.org/10.1210/jc.2014-2700
Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM, Pivonello R (2019) Acrom Nat Rev Dis Primers 5(1):20. https://doi.org/10.1038/s41572-019-0071-6
Carlsen SM, Lund-Johansen M, Schreiner T, Aanderud S, Johannesen O, Svartberg J, Cooper JG, Hald JK, Fougner SL, Bollerslev J (2008) Preoperative octreotide treatment in newly diagnosed acromegalic patients with macroadenomas increases cure short-term postoperative rates: a prospective, randomized trial. J Clin Endocrinol Metab 93(8):2984–2990. https://doi.org/10.1210/jc.2008-0315
Mao ZG, Zhu YH, Tang HL, Wang DY, Zhou J, He DS, Lan H, Luo BN, Wang HJ (2010) Preoperative lanreotide treatment in acromegalic patients with macroadenomas increases short-term postoperative cure rates: a prospective, randomised trial. Eur J Endocrinol 162(4):661–666. https://doi.org/10.1530/eje-09-0908
Mercado M, Borges F, Bouterfa H, Chang TC, Chervin A, Farrall AJ, Patocs A, Petersenn S, Podoba J, Safari M, Wardlaw J (2007) A prospective, multicentre study to investigate the efficacy, safety and tolerability of octreotide LAR (long-acting repeatable octreotide) in the primary therapy of patients with acromegaly. Clin Endocrinol 66(6):859–868. https://doi.org/10.1111/j.1365-2265.2007.02825.x
Melmed S, Cook D, Schopohl J, Goth MI, Lam KS, Marek J (2010) Rapid and sustained reduction of serum growth hormone and insulin-like growth factor-1 in patients with acromegaly receiving lanreotide Autogel therapy: a randomized, placebo-controlled, multicenter study with a 52 week open extension. Pituitary 13(1):18–28. https://doi.org/10.1007/s11102-009-0191-1
Bakhtiar Y, Hirano H, Arita K, Yunoue S, Fujio S, Tominaga A, Sakoguchi T, Sugiyama K, Kurisu K, Yasufuku-Takano J, Takano K (2010) Relationship between cytokeratin staining patterns and clinico-pathological features in somatotropinomae. Eur J Endocrinol 163(4):531–539. https://doi.org/10.1530/eje-10-0586
Bhayana S, Booth GL, Asa SL, Kovacs K, Ezzat S (2005) The implication of somatotroph adenoma phenotype to somatostatin analog responsiveness in acromegaly. J Clin Endocrinol Metab 90(11):6290–6295. https://doi.org/10.1210/jc.2005-0998
Paragliola RM, Corsello SM, Salvatori R (2017) Somatostatin receptor ligands in acromegaly: clinical response and factors predicting resistance. Pituitary 20(1):109–115. https://doi.org/10.1007/s11102-016-0768-4
Obari A, Sano T, Ohyama K, Kudo E, Qian ZR, Yoneda A, Rayhan N, Mustafizur Rahman M, Yamada S (2008) Clinicopathological features of growth hormone-producing pituitary adenomas: difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr Pathol 19(2):82–91. https://doi.org/10.1007/s12022-008-9029-Z
Heck A, Emblem KE, Casar-Borota O, Bollerslev J, Ringstad G (2016) Quantitative analyses of T2-weighted MRI as a potential marker for response to somatostatin analogs in newly diagnosed acromegaly. Endocrine 52(2):333–343. https://doi.org/10.1007/s12020-015-0766-8
Heck A, Ringstad G, Fougner SL, Casar-Borota O, Nome T, Ramm-Pettersen J, Bollerslev J (2012) Intensity of pituitary adenoma on T2-weighted magnetic resonance imaging predicts the response to octreotide treatment in newly diagnosed acromegaly. Clin Endocrinol 77(1):72–78. https://doi.org/10.1111/j.1365-2265.2011.04286.x
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577. https://doi.org/10.1148/radiol.2015151169
Park YW, Han K, Ahn SS, Choi YS, Chang JH, Kim SH, Kang SG, Kim EH, Lee SK (2018) Whole-tumor histogram and texture analyses of DTI for evaluation of IDH1-MUTATION and 1p/19q-codeletion status in World Health Organization Grade II Gliomas. Am J Neuroradiol 39(4):693–698. https://doi.org/10.3174/ajnr.A5569
Park YW, Choi YS, Ahn SS, Chang JH, Kim SH, Lee SK (2019) Radiomics MRI phenotyping with machine learning to predict the grade of lower-grade gliomas: a study focused on nonenhancing tumors. Korean J Radiol 20(9):1381–1389. https://doi.org/10.3348/kjr.2018.0814
Kickingereder P, Burth S, Wick A, Gotz M, Eidel O, Schlemmer HP, Maier-Hein KH, Wick W, Bendszus M, Radbruch A, Bonekamp D (2016) Radiomic profiling of glioblastoma: identifying an imaging predictor of patient survival with improved performance over established clinical and radiologic risk models. Radiology 280(3):880–889. https://doi.org/10.1148/radiol.2016160845
Zhang S, Song G, Zang Y, Jia J, Wang C, Li C, Tian J, Dong D, Zhang Y (2018) Non-invasive radiomics approach potentially predicts non-functioning pituitary adenomas subtypes before surgery. Eur Radiol 28(9):3692–3701. https://doi.org/10.1007/s00330-017-5180-6
Peng A, Dai H, Duan H, Chen Y, Huang J, Zhou L, Chen L (2020) A machine learning model to precisely immunohistochemically classify pituitary adenoma subtypes with radiomics based on preoperative magnetic resonance imaging. Eur J Radiol 125:108892. https://doi.org/10.1016/j.ejrad.2020.108892
Fan Y, Liu Z, Hou B, Li L, Liu X, Liu Z, Wang R, Lin Y, Feng F, Tian J, Feng M (2019) Development and validation of an MRI-based radiomic signature for the preoperative prediction of treatment response in patients with invasive functional pituitary adenoma. Eur J Radiol 121:108647. https://doi.org/10.1016/j.ejrad.2019.108647
Fan Y, Jiang S, Hua M, Feng S, Feng M, Wang R (2019) Machine learning-based radiomics predicts radiotherapeutic response in patients with acromegaly. Front Endocrinol 10:588
Lopes MBS (2017) The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol 134(4):521–535. https://doi.org/10.1007/s00401-017-1769-8
Cancer IAfRo (2017) WHO classification of tumours of endocrine organs, 4 edn. IARC
Vallières M, Freeman CR, Skamene SR, El Naqa I (2015) A radiomics model from joint FDG-PET and MRI texture features for the prediction of lung metastases in soft-tissue sarcomas of the extremities. Phys Med Biol 60(14):5471–5496. https://doi.org/10.1088/0031-9155/60/14/5471
Avants BB, Tustison N, Song G (2009) Advanced normalization tools (ANTS). Insight J 2(365):1–35
van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts H (2017) Computational radiomics system to decode the radiographic phenotype. Can Res 77(21):e104–e107. https://doi.org/10.1158/0008-5472.Can-17-0339
Zwanenburg A, Leger S, Vallières M, Löck S (2016) Image biomarker standardisation initiative. arXiv preprint arXiv:1612.07003
Hepp T, Schmid M, Gefeller O, Waldmann E, Mayr A (2016) Approaches to regularized regression: a comparison between gradient boosting and the lasso. Methods Inf Med 55(5):422–430. https://doi.org/10.3414/me16-01-0033
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Limkin EJ, Sun R, Dercle L, Zacharaki EI, Robert C, Reuzé S, Schernberg A, Paragios N, Deutsch E, Ferté C (2017) Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann Oncol 28(6):1191–1206. https://doi.org/10.1093/annonc/mdx034
Hagiwara A, Inoue Y, Wakasa K, Haba T, Tashiro T, Miyamoto T (2003) Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology 228(2):533–538. https://doi.org/10.1148/radiol.2282020695
Yamada S, Aiba T, Sano T, Kovacs K, Shishiba Y, Sawano S, Takada K (1993) Growth hormone-producing pituitary adenomas: correlations between clinical characteristics and morphology. Neurosurgery 33(1):20–27. https://doi.org/10.1227/00006123-199307000-00003
Horvath E, Kovacs K (2006) Pathology of acromegaly. Neuroendocrinology 83(3–4):161–165. https://doi.org/10.1159/000095524
Shen M, Zhang Q, Liu W, Wang M, Zhu J, Ma Z, He W, Li S, Shou X, Li Y, Zhang Z, Ye H, He M, Lu B, Yao Z, Lu Y, Qiao N, Ye Z, Zhang Y, Yang Y, Zhao Y, Wang Y (2016) Predictive value of T2 relative signal intensity for response to somatostatin analogs in newly diagnosed acromegaly. Neuroradiology 58(11):1057–1065. https://doi.org/10.1007/s00234-016-1728-4
Alhambra-Expósito MR, Ibáñez-Costa A, Moreno-Moreno P, Rivero-Cortés E, Vázquez-Borrego MC, Blanco-Acevedo C, Toledano-Delgado Á, Lombardo-Galera MS, Vallejo-Casas JA, Gahete MD, Castaño JP, Gálvez MA, Luque RM (2018) Association between radiological parameters and clinical and molecular characteristics in human somatotropinomas. Sci Rep 8(1):6173. https://doi.org/10.1038/s41598-018-24260-y
Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M (2013) Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: a large single center experience. Pituitary 16(4):490–498. https://doi.org/10.1007/s11102-012-0445-1
Ugga L, Cuocolo R, Solari D, Guadagno E, D'Amico A, Somma T, Cappabianca P, Del Basso de Caro ML, Cavallo LM, Brunetti A (2019) Prediction of high proliferative index in pituitary macroadenomas using MRI-based radiomics and machine learning. Neuroradiology 61(12):1365–1373
Cuocolo R, Ugga L, Solari D, Corvino S, D'Amico A, Russo D, Cappabianca P, Cavallo LM, Elefante A (2020) Prediction of pituitary adenoma surgical consistency: radiomic data mining and machine learning on T2-weighted MRI. Neuroradiology. https://doi.org/10.1007/s00234-020-02502-z
Fan Y, Chai Y, Li K, Fang H, Mou A, Feng S, Feng M, Wang R (2020) Non-invasive and real-time proliferative activity estimation based on a quantitative radiomics approach for patients with acromegaly: a multicenter study. J Endocrinol Invest 43(6):755–765. https://doi.org/10.1007/s40618-019-01159-7
Gatenby RA, Grove O, Gillies RJ (2013) Quantitative imaging in cancer evolution and ecology. Radiology 269(1):8–15. https://doi.org/10.1148/radiol.13122697
Fan M, Cheng H, Zhang P, Gao X, Zhang J, Shao G, Li L (2018) DCE-MRI texture analysis with tumor subregion partitioning for predicting Ki-67 status of estrogen receptor-positive breast cancers. J Magn Reson Imaging 48(1):237–247. https://doi.org/10.1002/jmri.25921
Fan M, Zhang P, Wang Y, Peng W, Wang S, Gao X, Xu M, Li L (2019) Radiomic analysis of imaging heterogeneity in tumours and the surrounding parenchyma based on unsupervised decomposition of DCE-MRI for predicting molecular subtypes of breast cancer. Eur Radiol 29(8):4456–4467. https://doi.org/10.1007/s00330-018-5891-3
Xu H, Lv W, Feng H, Du D, Yuan Q, Wang Q, Dai Z, Yang W, Feng Q, Ma J, Lu L (2019) Subregional radiomics analysis of PET/CT imaging with intratumor partitioning: application to prognosis for nasopharyngeal carcinoma. Mol Imag Biol. https://doi.org/10.1007/s11307-019-01439-x
Kocak B, Durmaz ES, Kadioglu P, Polat Korkmaz O, Comunoglu N, Tanriover N, Kocer N, Islak C, Kizilkilic O (2019) Predicting response to somatostatin analogues in acromegaly: machine learning-based high-dimensional quantitative texture analysis on T2-weighted MRI. Eur Radiol 29(6):2731–2739. https://doi.org/10.1007/s00330-018-5876-2
Galm BP, Buckless C, Swearingen B, Torriani M, Klibanski A, Bredella MA, Tritos NA (2020) MRI texture analysis in acromegaly and its role in predicting response to somatostatin receptor ligands. Pituitary 23(3):212–222. https://doi.org/10.1007/s11102-019-01023-0
O'Connor JP, Rose CJ, Waterton JC, Carano RA, Parker GJ, Jackson A (2015) Imaging intratumor heterogeneity: role in therapy response, resistance, and clinical outcome. Clin Cancer Res 21(2):249–257. https://doi.org/10.1158/1078-0432.Ccr-14-0990
Inoshita N, Nishioka H (2018) The 2017 WHO classification of pituitary adenoma: overview and comments. Brain Tumor Pathol 35(2):51–56. https://doi.org/10.1007/s10014-018-0314-3
Varma S, Simon R (2006) Bias in error estimation when using cross-validation for model selection. BMC Bioinform 7:91. https://doi.org/10.1186/1471-2105-7-91
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1I1A1A01071648).
Author information
Authors and Affiliations
Contributions
YWP and EHK designed the study. CRK, EHK, SHK, EJL, and SHK managed the patient recruitment and data acquisition. YK, SSA, and S-KL designed the radiomics pipeline and performed the radiomics analyses. YWP wrote the first draft of the manuscript and performed statistical analysis. EHK provided the critical revision of the manuscript. SHK supervised the manuscript. All authors contributed to and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethics approval
The Yonsei University Institutional Review Board waived the need for obtaining informed patient consent for this retrospective study. All methods were carried out in accordance with relevant guidelines and regulation.
Consent to participate
Informed consent was waived by the IRB due to the determination of minimal risk of the study.
Consent for publication
All authors agreed for publication of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, Y.W., Kang, Y., Ahn, S.S. et al. Radiomics model predicts granulation pattern in growth hormone-secreting pituitary adenomas. Pituitary 23, 691–700 (2020). https://doi.org/10.1007/s11102-020-01077-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-020-01077-5