Abstract
Purpose
To report patients with sellar tumors and chiasmal compression with normal visual fields, who demonstrate damage to the retinal nerve fiber layer (RNFL) and ganglion cell complex (GCC) on optical coherence tomography (OCT).
Methods
Seven patients with sellar tumors causing mass effect on the optic chiasm without definite visual field defect, but abnormal GCC are described. GCC/RNFL analyses using Cirrus-OCT were classified into centiles based on the manufacturer’s reference range.
Results
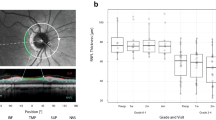
In seven patients with radiologic compression of the chiasm by a sellar tumor, OCT-GCC thickness detected compressive chiasmopathy before visual defects became apparent on standard automated visual field testing. Without OCT, our patients would have been labelled as having normal visual function and no evidence of compressive chiasmopathy. With only OCT-RNFL analysis, 3/7 patients would still have been labelled as having no compression of the anterior visual pathways.
Conclusions
These patients show that OCT-GCC analysis is more sensitive than visual field testing with standard automated perimetry in the detection of compressive chiasmopathy or optic neuropathy. These cases and previous studies suggest that OCT-GCC analysis may be used in addition to visual field testing to evaluate patients with lesions compressing the chiasm.



Similar content being viewed by others
References
Casanueva FF, Barkan AL, Buchfelder M, Klibanski A, Laws ER, Loeffler JS, Melmed S, Mortini P, Wass J, Giustina A (2017) Pituitary society, expert group on pituitary tumors. Criteria for the definition of pituitary tumor centers of excellence (PTCOE): a pituitary society statement. Pituitary 20:489–498
Ryu WHA, Starreveld Y, Burton JM, Liu J, Costello F, Group PS (2017) The utility of magnetic resonance imaging in assessing patients with pituitary tumors compressing the anterior visual pathway. J Neuroophthalmol 37:230–238
Newman SA, Turbin RE, Bodach ME, Tumialan LM, Oyesiku NM, Litvack Z, Zada G, Patil CG, Aghi MK (2016) Congress of neurological surgeons systematic review and evidence-based guideline on pretreatment ophthalmology evaluation in patients with suspected nonfunctioning pituitary adenomas. Neurosurgery 79:E530–E532
Kedar S, Ghate D, Corbett JJ (2011) Visual fields in neuro-ophthalmology. Indian J Ophthalmol 59:103–109
Danesh-Meyer HV, Carroll SC, Foroozan R, Savino PJ, Fan J, Jiang Y, Vander Hoorn S (2006) Relationship between retinal nerve fiber layer and visual field sensitivity as measured by optical coherence tomography in chiasmal compression. Invest Ophthalmol Vis Sci 47:4827–4835
Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD (2008) In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci 49:1879–1885
Phal PM, Steward C, Nichols AD, Kokkinos C, Desmond PM, Danesh-Meyer H, Sufaro YZ, Kaye AH, Moffat BA (2016) Assessment of optic pathway structure and function in patients with compression of the optic chiasm: a correlation with optical coherence tomography. Invest Ophthalmol Vis Sci 57:3884–3890
Jacob M, Raverot G, Jouanneau E, Borson-Chazot F, Perrin G, Rabilloud M, Tilikete C, Bernard M, Vighetto A (2009) Predicting visual outcome after treatment of pituitary adenomas with optical coherence tomography. Am J Ophthalmol 147:64–70 e62
Loo JL, Tian J, Miller NR, Subramanian PS (2013) Use of optical coherence tomography in predicting post-treatment visual outcome in anterior visual pathway meningiomas. Br J Ophthalmol 97:1455–1458
Monteiro ML, Hokazono K, Fernandes DB, Costa-Cunha LV, Sousa RM, Raza AS, Wang DL, Hood DC (2014) Evaluation of inner retinal layers in eyes with temporal hemianopic visual loss from chiasmal compression using optical coherence tomography. Invest Ophthalmol Vis Sci 55:3328–3336
Danesh-Meyer HV, Wong A, Papchenko T, Matheos K, Stylli S, Nichols A, Frampton C, Daniell M, Savino PJ, Kaye AH (2015) Optical coherence tomography predicts visual outcome for pituitary tumors. J Clin Neurosci 22:1098–1104
Johansson C, Lindblom B (2009) The role of optical coherence tomography in the detection of pituitary adenoma. Acta Ophthalmol 87:776–779
Tieger MG, Hedges TR III, Ho J, Erlich-Malona NK, Vuong LN, Athappilly GK, Mendoza-Santiesteban CE (2017) Ganglion cell complex loss in chiasmal compression by brain tumors. J Neuroophthalmol 37:7–12
Yum HR, Park SH, Park HY, Shin SY (2016) Macular ganglion cell analysis determined by cirrus HD optical coherence tomography for early detecting chiasmal compression. PLoS ONE 11:e0153064
Monteiro ML, Costa-Cunha LV, Cunha LP, Malta RF (2010) Correlation between macular and retinal nerve fibre layer Fourier-domain OCT measurements and visual field loss in chiasmal compression. Eye 24:1382–1390
Akashi A, Kanamori A, Ueda K, Matsumoto Y, Yamada Y, Nakamura M (2014) The detection of macular analysis by SD-OCT for optic chiasmal compression neuropathy and nasotemporal overlap. Invest Ophthalmol Vis Sci 55:4667–4672
Moon CH, Hwang SC, Ohn YH, Park TK (2011) The time course of visual field recovery and changes of retinal ganglion cells after optic chiasmal decompression. Invest Ophthalmol Vis Sci 52:7966–7973
Horton JC (2017) Invited commentary: ganglion cell complex measurement in compressive optic neuropathy. J Neuroophthalmol 37:13–15
Jeong AR, Kim EY, Kim NR (2016) Preferential ganglion cell loss in the nasal hemiretina in patients with pituitary tumor. J Neuroophthalmol 36:152–155
Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R (2013) Glaucomatous damage of the macula. Prog Retin Eye Res 32:1–21
Vuong LN, Hedges TR III (2017) Ganglion cell layer complex measurements in compressive optic neuropathy. Curr Opin Ophthalmol 28:573–578
Zehnder S, Wildberger H, Hanson JVM, Lukas S, Pelz S, Landau K, Wichmann W, Gerth-Kahlert C (2018) Retinal ganglion cell topography in patients with visual pathway pathology. J Neuroophthalmol 38:172–178
Azuara-Blanco A, Banister K, Boachie C, McMeekin P, Gray J, Burr J, Bourne R, Garway-Heath D, Batterbury M, Hernández R, McPherson G, Ramsay C, Cook J (2016) Automated imaging technologies for the diagnosis of glaucoma: a comparative diagnostic study for the evaluation of the diagnostic accuracy, performance as triage tests and cost-effectiveness (GATE study). Health Technol Assess 20:1–168
Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS (2000) Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci 41:741–748
Ferreras A, Polo V, Larrosa JM, Pablo LE, Pajarin AB, Pueyo V, Honrubia FM (2007) Can frequency-doubling technology and short-wavelength automated perimetries detect visual field defects before standard automated perimetry in patients with preperimetric glaucoma? J Glaucoma 16:372–383
Wall M, Neahring RK, Woodward KR (2002) Sensitivity and specificity of frequency doubling perimetry in neuro-ophthalmic disorders: a comparison with conventional automated perimetry. Invest Ophthalmol Vis Sci 43:1277–1283
Kim KE, Jeoung JW, Kim DM, Ahn SJ, Park KH, Kim SH (2015) Long-term follow-up in preperimetric open-angle glaucoma: progression rates and associated factors. Am J Ophthalmol 159:160–168
Chen JJ, Kardon RH (2016) Avoiding clinical misinterpretation and artifacts of optical coherence tomography analysis of the optic nerve, retinal nerve fiber layer, and ganglion cell layer. J Neuroophthalmol 36:417–438
Funding
This work was supported in part by an unrestricted departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc., New York, and by NIH/NEI core Grant P30-EY006360 (Department of Ophthalmology). Dr. Biousse received research support from NIH/PHS (UL1-RR025008). Dr. Newman is a recipient of the Research to Prevent Blindness Lew R. Wasserman Merit Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicting relationship exists for any author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blanch, R.J., Micieli, J.A., Oyesiku, N.M. et al. Optical coherence tomography retinal ganglion cell complex analysis for the detection of early chiasmal compression. Pituitary 21, 515–523 (2018). https://doi.org/10.1007/s11102-018-0906-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-018-0906-2