Abstract
Purpose
Initial successful surgical treatment of pituitary adenomas is crucial to reach long-term remission. Indocyanine green (ICG) videoangiography (VA) is well established in vascular neurosurgery nowadays and several reports described ICG application in brain tumor surgery. We designed this study to evaluate the feasibility of intravenous application of ICG and visualisation of a pituitary lesion via the fluorescence mode of the operation microscope.
Methods
22 patients with pituitary adenomas were treated with transsphenoidal microsurgery and were included in this study. Intraoperatively 25 mg ICG was administered intravenously and visualized via the fluorescence mode of the operation microscope (Pentero/Zeiss).
Results
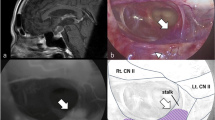
22 patients qualified for transsphenoidal surgery presenting with different clinical symptoms (13 patients with acromegaly, 6 with M. Cushing and 3 with other symptoms like vision disorder or dizziness) and identification of a pituitary lesion (21 of 22 patients) in preoperative MR-imaging (mean diameter: 9 mm; SD 3.6; 6 macroadenomas, 15 microadenomas, 1 MR-negative). In all 22 patients ICG VA was performed during surgery. No technical failures or adverse events after drug administration occurred. Visualization was optimal approximately 2.4 min after intravenous application. In all patients the adenoma could be detected via two different types of visualization: direct visualization by fluorophore emission versus indirect detection of the adenoma by a lower ICG fluorescence compared to the surrounding tissue.
Conclusion
Our data show that intraoperative ICG VA can be a useful and easily applicable additional diagnostic tool for visualization of pituitary lesions using the microscopic approach.


Similar content being viewed by others
References
Hameed N, Yedinak CG, Brzana J, Gultekin SH, Coppa ND, Dogan A et al (2013) Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary 16(4):452–458
Pieters GF, Hermus AR, Meijer E, Smals AG, Kloppenborg PW (1989) Predictive factors for initial cure and relapse rate after pituitary surgery for Cushing’s disease. J Clin Endocrinol Metab 69(6):1122–1126
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP et al (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88(12):5593–5602
Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF (2002) Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clin Endocrinol 56(4):541–551
Hazer DB, Isik S, Berker D, Guler S, Gurlek A, Yucel T et al (2013) Treatment of acromegaly by endoscopic transsphenoidal surgery: surgical experience in 214 cases and cure rates according to current consensus criteria. J Neurosurg 119(6):1467–1477
Acebes JJ, Martino J, Masuet C, Montanya E, Soler J (2007) Early post-operative ACTH and cortisol as predictors of remission in Cushing’s disease. Acta Neurochir (Wien) 149(5):471–477 Discussion 7–9
Tanei T, Nagatani T, Nakahara N, Watanabe T, Nishihata T, Nielsen ML et al (2013) Use of high-field intraoperative magnetic resonance imaging during endoscopic transsphenoidal surgery for functioning pituitary microadenomas and small adenomas located in the intrasellar region. Neurol Med Chir 53(7):501–510
Woitzik J, Horn P, Vajkoczy P, Schmiedek P (2005) Intraoperative control of extracranial–intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg 102(4):692–698
de Oliveira JG, Beck J, Seifert V, Teixeira MJ, Raabe A (2008) Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery 62(6 Suppl 3):1300–1310
Imizu S, Kato Y, Sangli A, Oguri D, Sano H (2008) Assessment of incomplete clipping of aneurysms intraoperatively by a near-infrared indocyanine green-video angiography (Niicg-Va) integrated microscope. Minim Invasive Neurosurg 51(4):199–203
Hanggi D, Etminan N, Steiger HJ (2010) The impact of microscope-integrated intraoperative near-infrared indocyanine green videoangiography on surgery of arteriovenous malformations and dural arteriovenous fistulae. Neurosurgery 67(4):1094–1103 Discussion 103–104
Kim EH, Cho JM, Chang JH, Kim SH, Lee KS (2011) Application of intraoperative indocyanine green videoangiography to brain tumor surgery. Acta Neurochir (Wien) 153(7):1487–1495 Discussion 94-5
Murai Y, Adachi K, Matano F, Tateyama K, Teramoto A (2011) Indocyanin green videoangiography study of hemangioblastomas. Can J Neurol Sci 38(1):41–47
Ferroli P, Acerbi F, Albanese E, Tringali G, Broggi M, Franzini A et al (2011) Application of intraoperative indocyanine green angiography for CNS tumors: results on the first 100 cases. Acta Neurochir Suppl 109:251–257
Litvack ZN, Zada G, Laws ER Jr (2012) Indocyanine green fluorescence endoscopy for visual differentiation of pituitary tumor from surrounding structures. J Neurosurg 116(5):935–941
Jugenburg M, Kovacs K, Stefaneanu L, Scheithauer BW (1995) Vasculature in nontumorous hypophyses, pituitary adenomas, and carcinomas: a quantitative morphologic study. Endocr Pathol 6(2):115–124
Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA (2000) Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab 85(3):1159–1162
Yamada S, Takada K (2003) Angiogenesis in pituitary adenomas. Microsc Res Tech 60(2):236–243
Viacava P, Gasperi M, Acerbi G, Manetti L, Cecconi E, Bonadio AG et al (2003) Microvascular density and vascular endothelial growth factor expression in normal pituitary tissue and pituitary adenomas. J Endocrinol Invest 26(1):23–28
Niveiro M, Aranda FI, Peiro G, Alenda C, Pico A (2005) Immunohistochemical analysis of tumor angiogenic factors in human pituitary adenomas. Hum Pathol 36(10):1090–1095
Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA (2000) Angiogenesis in pituitary adenomas—relationship to endocrine function, treatment and outcome. J Endocrinol 165(2):475–481
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93(6):1003–1013
Nokes B, Apel M, Jones C, Brown G, Lang JE (2013) Aminolevulinic acid (ALA): photodynamic detection and potential therapeutic applications. J Surg Res 181(2):262–271
Paterno V, Fahlbusch R (2014) High-field iMRI in transsphenoidal pituitary adenoma surgery with special respect to typical localization of residual tumor. Acta Neurochir (Wien) 156(3):463–474 Discussion 74
Sylvester PT, Evans JA, Zipfel GJ, Chole RA, Uppaluri R, Haughey BH et al (2014) Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free survival for pituitary adenomas. Pituitary. doi:10.1007/s11102-014-0560-2.
Buchfelder M, Schlaffer SM (2012) Intraoperative magnetic resonance imaging during surgery for pituitary adenomas: pros and cons. Endocrine 42(3):483–495
Hofmann BM, Hlavac M, Martinez R, Buchfelder M, Muller OA, Fahlbusch R (2008) Long-term results after microsurgery for Cushing disease: experience with 426 primary operations over 35 years. J Neurosurg 108(1):9–18
Acknowledgments
The authors are grateful to Mr. Manfred Eifler-Sander for clinical patient management and documentation.
Conflict of interest
All of the authors are aware of and agree to the content of the manuscript and their being listed as an author on the manuscript. None of the authors has any financial interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandow, N., Klene, W., Elbelt, U. et al. Intraoperative indocyanine green videoangiography for identification of pituitary adenomas using a microscopic transsphenoidal approach. Pituitary 18, 613–620 (2015). https://doi.org/10.1007/s11102-014-0620-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-014-0620-7