Abstract
Purpose
The clinical benefit of combined intraoperative magnetic resonance imaging (iMRI) and endoscopy for transsphenoidal pituitary adenoma resection has not been completely characterized. This study assessed the impact of microscopy, endoscopy, and/or iMRI on progression-free survival, extent of resection status (gross-, near-, and sub-total resection), and operative complications.
Methods
Retrospective analyses were performed on 446 transsphenoidal pituitary adenoma surgeries at a single institution between 1998 and 2012. Multivariate analyses were used to control for baseline characteristics, differences during extent of resection status, and progression-free survival analysis.
Results
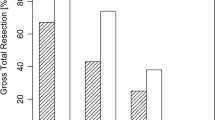
Additional surgery was performed after iMRI in 56/156 cases (35.9 %), which led to increased extent of resection status in 15/156 cases (9.6 %). Multivariate ordinal logistic regression revealed no increase in extent of resection status following iMRI or endoscopy alone; however, combining these modalities increased extent of resection status (odds ratio 2.05, 95 % CI 1.21–3.46) compared to conventional transsphenoidal microsurgery. Multivariate Cox regression revealed that reduced extent of resection status shortened progression-free survival for near- versus gross-total resection [hazard ratio (HR) 2.87, 95 % CI 1.24–6.65] and sub- versus near-total resection (HR 2.10; 95 % CI 1.00–4.40). Complication comparisons between microscopy, endoscopy, and iMRI revealed increased perioperative deaths for endoscopy versus microscopy (4/209 and 0/237, respectively), but this difference was non-significant considering multiple post hoc comparisons (Fisher exact, p = 0.24).
Conclusions
Combined use of endoscopy and iMRI increased pituitary adenoma extent of resection status compared to conventional transsphenoidal microsurgery, and increased extent of resection status was associated with longer progression-free survival. Treatment modality combination did not significantly impact complication rate.




Similar content being viewed by others
References
Chang EF, Zada G, Kim S et al (2008) Long-term recurrence and mortality after surgery and adjuvant radiotherapy for nonfunctional pituitary adenomas. J Neurosurg 108:736–745
Goudakos JK, Markou KD, Georgalas C (2011) Endoscopic versus microscopic trans-sphenoidal pituitary surgery: a systematic review and meta-analysis. Clin Otolaryngol 36:212–220
Tabaee A, Anand VK, Barrón Y et al (2009) Endoscopic pituitary surgery: a systematic review and meta-analysis. J Neurosurg 111:545–554
Cho D-Y, Liau W-R (2002) Comparison of endonasal endoscopic surgery and sublabial microsurgery for prolactinomas. Surg Neurol 58:371–375
Chole RA, Lim C, Dunham B et al (2011) A novel transnasal transsphenoidal speculum: a design for both microscopic and endoscopic transsphenoidal pituitary surgery. J Neurosurg 114:1380–1385
D’Haens J, Van Rompaey K, Stadnik T et al (2009) Fully endoscopic transsphenoidal surgery for functioning pituitary adenomas: a retrospective comparison with traditional transsphenoidal microsurgery in the same institution. Surg Neurol 72:336–340
McLaughlin N, Eisenberg AA, Cohan P et al (2013) Value of endoscopy for maximizing tumor removal in endonasal transsphenoidal pituitary adenoma surgery. J Neurosurg 118:613–620
Messerer M, De Battista JC, Raverot G et al (2011) Evidence of improved surgical outcome following endoscopy for nonfunctioning pituitary adenoma removal. Neurosurg Focus 30:E11
Ammirati M, Wei L, Ciric I (2013) Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 84:843–849
Starke RM, Raper DMS, Payne SC et al (2013) Endoscopic versus microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab 98:1–10
Anand VK, Schwartz TH, Hiltzik DH, Kacker A (2006) Endoscopic transsphenoidal pituitary surgery with real-time intraoperative magnetic resonance imaging. Am J Rhinol 20:401–405
Berkmann S, Fandino J, Zosso S et al (2011) Intraoperative magnetic resonance imaging and early prognosis for vision after transsphenoidal surgery for sellar lesions. J Neurosurg 115:518–527
Fahlbusch R, Ganslandt O, Buchfelder M et al (2001) Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg 95:381–390
Gerlach R, du Mesnil de Rochemont R, Gasser T et al (2008) Feasibility of Polestar N20, an ultra-low-field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery 63:272–275
Schwartz TH, Stieg PE, Anand VK (2006) Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Neurosurgery 58:44–51
Vitaz TW, Inkabi KE, Carrubba CJ (2011) Intraoperative MRI for transsphenoidal procedures: short-term outcome for 100 consecutive cases. Clin Neurol Neurosurg 113:731–735
Wu JS, Shou XF, Yao CJ et al (2009) Transsphenoidal pituitary macroadenomas resection guided by PoleStar N20 low-field intraoperative magnetic resonance imaging: comparison with early postoperative high-field magnetic resonance imaging. Neurosurgery 65:61–63
Fahlbusch R, Keller B, Ganslandt O et al (2005) Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur J Endocrinol 153:239–248
Netuka D, Masopust V, Belsan T et al (2011) One year experience with 3.0 T intraoperative MRI in pituitary surgery. Acta Neurochir Suppl 109:3–5
Nimsky C, Ganslandt O, Fahlbusch R (2005) Comparing 0.2 tesla with 1.5 tesla intraoperative magnetic resonance imaging analysis of setup, workflow, and efficiency. Acad Radiol 12:1065–1079
Nimsky C, Ganslandt O, Von Keller B et al (2004) Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology 233:67–78
Nimsky C, Keller B, Ganslandt O et al (2006) Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery 58:105–114
Pamir MN, Peker S, Ozek MM, Dincer A (2006) Intraoperative MR imaging: preliminary results with 3 tesla MR system. Acta Neurochir Suppl 98:97–100
Szerlip NJ, Zhang Y-CC, Placantonakis DG et al (2011) Transsphenoidal resection of sellar tumors using high-field intraoperative magnetic resonance imaging. Skull Base 21:223–232
Coburger J, Konig R, Seitz K, et al (2014) Determining the utility of intraoperative magnetic resonance imaging for transsphenoidal surgery: a retrospective study. J Neurosurg 120:346–356
Chicoine MR, Lim CC, Evans JA et al (2011) Implementation and preliminary clinical experience with the use of ceiling mounted mobile high field intraoperative magnetic resonance imaging between two operating rooms. Acta Neurochir Suppl 109:97–102
Hall WA, Kowalik K, Liu H et al (2003) Costs and benefits of intraoperative MR-guided brain tumor resection. Acta Neurochir Suppl 85:137–142
Theodosopoulos PV, Leach J, Kerr RG et al (2010) Maximizing the extent of tumor resection during transsphenoidal surgery for pituitary macroadenomas: can endoscopy replace intraoperative magnetic resonance imaging? J Neurosurg 112:736–743
Chicoine RM, Evans AJ, Wippold JF et al (2011) Comparison of intraoperative and postoperative MRI for endoscopic transsphenoidal resection of pituitary macroadenomas. Skull Base 21:A064
Haydon DH, Chicoine MR, Dacey RG (2013) The impact of high-field-strength intraoperative magnetic resonance imaging on brain tumor management. Neurosurgery 60:92–97
Leuthardt EC, Lim CCH, Shah MN et al (2011) Use of movable high-field-strength intraoperative magnetic resonance imaging with awake craniotomies for resection of gliomas: preliminary experience. Neurosurgery 69:194–205
Shah MN, Leonard JR, Inder G et al (2012) Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr 9:259–264
De Paiva Neto MA, Vandergrift A, Fatemi N et al (2010) Endonasal transsphenoidal surgery and multimodality treatment for giant pituitary adenomas. Clin Endocrinol 72:512–519
Di Maio S, Cavallo LM, Esposito F et al (2011) Extended endoscopic endonasal approach for selected pituitary adenomas: early experience. J Neurosurg 114:345–353
Arnaldi G, Angeli A, Atkinson A et al (2003) Diagnosis and complications of Cushing’s Syndrome: a consensus statement. J Clin Endocrinol Metab 88:5593–5602
Casanueva FF, Molitch ME, Schlechte JA et al (2006) Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol 65:265–273
Giustina A, Chanson P, Bronstein MD et al (2010) A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab 95:3141–3148
Ekramullah SM, Saitoh Y, Arita N et al (1996) The correlation of Ki-67 staining indices with tumour doubling times in regrowing non-functioning pituitary adenomas. Acta Neurochir 138:1449–1455
Green VL, Atkin SL, Speirs V et al (1996) Cytokine expression in human anterior pituitary adenomas. Clin Endocrinol 45:179–185
Tanaka Y, Hongo K, Tada T et al (2003) Growth pattern and rate in residual nonfunctioning pituitary adenomas: correlations among tumor volume doubling time, patient age, and MIB-1 index. J Neurosurg 98:359–365
Buchfelder M, Schlaffer S-M (2012) Intraoperative magnetic resonance imaging during surgery for pituitary adenomas: pros and cons. Endocrine 42:483–495
Lang MJ, Kelly JJ, Sutherland GR (2011) A moveable 3-Tesla intraoperative magnetic resonance imaging system. Neurosurgery 68:168–179
Koutourousiou M, Gardner PA, Fernandez-Miranda JC et al (2013) Endoscopic endonasal surgery for giant pituitary adenomas: advantages and limitations. J Neurosurg 118:621–631
Losa M, Mortini P, Barzaghi R et al (2008) Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg 108:525–532
Berker M, Hazer DB, Yücel T et al (2012) Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary 15:288–300
Mortini P, Losa M, Barzaghi R et al (2005) Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery 56:1222–1233
Frank G, Pasquini E, Farneti G et al (2006) The endoscopic versus the traditional approach in pituitary surgery. Neuroendocrinology 83:240–248
Kabil MS, Eby JB, Shahinian HK (2005) Fully endoscopic endonasal vs. transseptal transsphenoidal pituitary surgery. Minim Invasive Neurosurg 48:348–354
Halvorsen H, Ramm-Pettersen J, Josefsen R, et al. (2013) Surgical complications after transsphenoidal microscopic and endoscopic surgery for pituitary adenoma: a consecutive series of 506 procedures. Acta Neurochir (Wien) Epub before print
Jane JJ, Laws E (2001) The surgical management of pituitary adenomas in a series of 3,093 patients. J Am Coll Surg 193:651–659
DeKlotz TR, Chia SH, Lu W et al (2012) Meta-analysis of endoscopic versus sublabial pituitary surgery. Laryngoscope 122:511–518
Ciric I, Ragin A, Baumgartner C, Pierce D (1997) Complications of transsphenoidal surgery: results of a national survey, review of the literature, and personal experience. Neurosurgery 40:225–236
Senft C, Bink A, Franz K et al (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12:997–1003
Stummer W (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62:564–576
Acknowledgments
We would like to thank Feng Gao, PhD and J. Phillip Miller, PhD from the Department of Biostatistics at Washington University in St. Louis for his advice regarding the statistical methods performed in this study. Furthermore, we would like to thank Bridget McCullough & Stan Goddard our MRI technologists, and Kathy Draege our neurosurgical operating room charge nurse and our entire operating room staff. These individuals enable the safe completion of these surgical procedures in the complex iMRI environment
Conflict of interest
Michael Chicoine and John Evans received funding from IMRIS Inc. for an unrestricted educational grant that has helped support the iMRI database and outcomes analysis. The authors have no personal financial or institutional interest in any of the drugs, materials, or devices described in this article. Peter Sylvester received grant support from the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Numbers UL1 TR000448 and TL1 TR000449.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sylvester, P.T., Evans, J.A., Zipfel, G.J. et al. Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free survival for pituitary adenomas. Pituitary 18, 72–85 (2015). https://doi.org/10.1007/s11102-014-0560-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-014-0560-2