Abstract
Purpose
It is becoming evident that long non-coding RNAs (lncRNAs) participate in diverse biological processes via distinct mechanisms. Many lncRNAs have altered expression and likely to have functional roles in tumorigenesis. Although loss of maternally-expressed gene 3 (MEG3) expression has been detected in non-functioning pituitary adenomas (NFPAs), there are no published reports regarding the association between MEG3 expression and the invasive ability of NFPAs. Moreover, the roles of Hox transcript antisense intergenic RNA (HOTAIR) and metastasis-associated lung adenocarcinoma transcript 1 (MALAT-1) in NFPAs have not been examined. To investigate the role of MEG3, HOTAIR, and MALAT-1 in NFPA development and invasion.
Methods
MEG3, HOTAIR, MALAT-1 and proliferating cell nuclear antigen (PCNA) were detected in 52 NFPA samples and seven normal human anterior pituitaries using real-time quantitative reverse transcription polymerase chain reaction.
Results
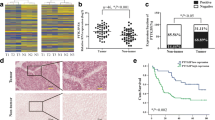
MEG3 lncRNA levels gradually decreased whereas HOTAIR lncRNA levels gradually increased from normal anterior pituitaries to non-invasive NFPAs to invasive NFPAs. There was a significant association between MEG3 (P < 0.01) and HOTAIR (P < 0.05) expression and the biological behavior of the tumor. Furthermore, PCNA mRNA levels markedly increased in invasive NFPAs compared to non-invasive ones (P < 0.01). In addition, PCNA mRNA negatively correlated with MEG3 lncRNA levels (P < 0.05).
Conclusions
MEG3 and HOTAIR expression may correlate with NFPA development and invasion.


Similar content being viewed by others
References
Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A (2006) High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab 91(12):4769–4775. doi:10.1210/jc.2006-1668
Fernandez A, Karavitaki N, Wass JA (2010) Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf) 72(3):377–382. doi:10.1111/j.1365-2265.2009.03667.x
Katznelson L, Alexander JM, Klibanski A (1993) Clinical review 45: clinically nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 76(5):1089–1094. doi:10.1210/jc.76.5.1089
Melmed S (2011) Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7(5):257–266. doi:10.1038/nrendo.2011.40
Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, Jacks T, Hacohen N, Bernstein BE, Kellis M, Regev A, Rinn JL, Lander ES (2009) Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458(7235):223–227. doi:10.1038/nature07672
Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136(4):629–641. doi:10.1016/j.cell.2009.02.006
Wapinski O, Chang HY (2011) Long noncoding RNAs and human disease. Trends Cell Biol 21(6):354–361. doi:10.1016/j.tcb.2011.04.001
Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Muller-Tidow C (2003) MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22(39):8031–8041. doi:10.1038/sj.onc.1206928
Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE (2010) Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet 6(12):e1001233. doi:10.1371/journal.pgen.1001233
Miyoshi N, Wagatsuma H, Wakana S, Shiroishi T, Nomura M, Aisaka K, Kohda T, Surani MA, Kaneko-Ishino T, Ishino F (2000) Identification of an imprinted gene, Meg3/Gtl2 and its human homologue MEG3, first mapped on mouse distal chromosome 12 and human chromosome 14q. Genes Cells 5(3):211–220
Zhou Y, Zhang X, Klibanski A (2012) MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol 48(3):R45–R53. doi:10.1530/jme-12-0008
Zhang X, Zhou Y, Mehta KR, Danila DC, Scolavino S, Johnson SR, Klibanski A (2003) A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. J Clin Endocrinol Metab 88(11):5119–5126
Bando T, Kato Y, Ihara Y, Yamagishi F, Tsukada K, Isobe M (1999) Loss of heterozygosity of 14q32 in colorectal carcinoma. Cancer Genet Cytogenet 111(2):161–165
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu WQ, Xie WP, Hou YY (2013) Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC Cancer 13(1):461. doi:10.1186/1471-2407-13-461
Braconi C, Kogure T, Valeri N, Huang N, Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM, Patel T (2011) microRNA-29 can regulate expression of the long non-coding RNA gene MEG3 in hepatocellular cancer. Oncogene 30(47):4750–4756. doi:10.1038/onc.2011.193
Wang P, Ren Z, Sun P (2012) Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J Cell Biochem 113(6):1868–1874. doi:10.1002/jcb.24055
Zhang X, Gejman R, Mahta A, Zhong Y, Rice KA, Zhou Y, Cheunsuchon P, Louis DN, Klibanski A (2010) Maternally expressed gene 3, an imprinted noncoding RNA gene, is associated with meningioma pathogenesis and progression. Cancer Res 70(6):2350–2358. doi:10.1158/0008-5472.can-09-3885
Wilson CB (1984) A decade of pituitary microsurgery The Herbert Olivecrona lecture. J Neurosurg 61(5):814–833. doi:10.3171/jns.1984.61.5.0814
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–617 discussion 617-618
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3(6):1101–1108
Cheunsuchon P, Zhou Y, Zhang X, Lee H, Chen W, Nakayama Y, Rice KA, Tessa Hedley-Whyte E, Swearingen B, Klibanski A (2011) Silencing of the imprinted DLK1-MEG3 locus in human clinically nonfunctioning pituitary adenomas. Am J Pathol 179(4):2120–2130. doi:10.1016/j.ajpath.2011.07.002
Gejman R, Batista DL, Zhong Y, Zhou Y, Zhang X, Swearingen B, Stratakis CA, Hedley-Whyte ET, Klibanski A (2008) Selective loss of MEG3 expression and intergenic differentially methylated region hypermethylation in the MEG3/DLK1 locus in human clinically nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 93(10):4119–4125. doi:10.1210/jc.2007-2633
Bravo R, Frank R, Blundell PA, Macdonald-Bravo H (1987) Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature 326(6112):515–517. doi:10.1038/326515a0
Zhao J, Dahle D, Zhou Y, Zhang X, Klibanski A (2005) Hypermethylation of the promoter region is associated with the loss of MEG3 gene expression in human pituitary tumors. J Clin Endocrinol Metab 90(4):2179–2186. doi:10.1210/jc.2004-1848
Zhou Y, Zhong Y, Wang Y, Zhang X, Batista DL, Gejman R, Ansell PJ, Zhao J, Weng C, Klibanski A (2007) Activation of p53 by MEG3 non-coding RNA. J Biol Chem 282(34):24731–24742. doi:10.1074/jbc.M702029200
Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129(7):1311–1323. doi:10.1016/j.cell.2007.05.022
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464(7291):1071–1076. doi:10.1038/nature08975
Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S (2013) HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 32(13):1616–1625. doi:10.1038/onc.2012.193
Nakagawa T, Endo H, Yokoyama M, Abe J, Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T, Satoh K (2013) Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun 436(2):319–324. doi:10.1016/j.bbrc.2013.05.101
Lin R, Maeda S, Liu C, Karin M, Edgington TS (2007) A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene 26(6):851–858. doi:10.1038/sj.onc.1209846
Guffanti A, Iacono M, Pelucchi P, Kim N, Solda G, Croft LJ, Taft RJ, Rizzi E, Askarian-Amiri M, Bonnal RJ, Callari M, Mignone F, Pesole G, Bertalot G, Bernardi LR, Albertini A, Lee C, Mattick JS, Zucchi I, De Bellis G (2009) A transcriptional sketch of a primary human breast cancer by 454 deep sequencing. BMC Genomics 10:163. doi:10.1186/1471-2164-10-163
Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV (2010) The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell 39(6):925–938. doi:10.1016/j.molcel.2010.08.011
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31000498).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Z., Li, C., Liu, C. et al. Expression of the long non-coding RNAs MEG3, HOTAIR, and MALAT-1 in non-functioning pituitary adenomas and their relationship to tumor behavior. Pituitary 18, 42–47 (2015). https://doi.org/10.1007/s11102-014-0554-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-014-0554-0