Abstract
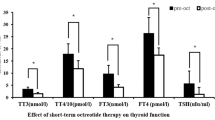
Surgical cure cannot be achieved in most patients with invasive non-functioning pituitary macroadenoma (NFPA). Short-term residual tumor treatment with somatostatin analogs has produced disappointing results. This prospective case–control study assessed the efficacy of chronic treatment with long acting octreotide (octreotide LAR) on tumor volume in patients harboring post-surgical NFPA residue. The study population comprised 39 patients with NFPAs not cured by surgery. All patients underwent somatostatin receptor scintigraphy at least 6 months after the last surgery. Patients with a positive pituitary level octreoscan at (n = 26) received octreotide LAR (20 mg every 28 days) for ≥12 months (mean follow-up 37 ± 18 months) (Treated group). Moreover, a fragment of tumor tissue from patients in the treated group was retrospectively collected to assess the immunohistochemical expression of somatostatin receptor subtypes (SSTRs). The patients with a negative octreoscan (n = 13) formed the control group (mean follow-up 37 ± 16 months). Hormonal, radiological and visual field parameters were periodically assessed. In the treated group, all tumors expressed at least one SSTR subtype. The SSTR5 subtype was the most abundant, followed by SSTR3. The tumor residue increased in five of 26 patients (19%) in the treated group and in seven of 13 controls (53%). Visual field and pituitary function did not change in any patient. This study indicates that SSTR5 and SSTR3 are the most frequently expressed SSTR subtypes in NFPAs and supports a potential role of SSTR subtypes in stabilization of tumor remnant from NFPAs.


Similar content being viewed by others
References
Reddy R, Cudlip S, Byrne JV, Karavitaki N, Wass JAH (2011) Can we ever stop imaging in surgically treated and radiotherapy-naive patients with non-functioning pituitary adenoma? Eur J Endocrinol 165:739–744
Jaffe CA (2006) Clinically non-functioning pituitary adenoma. Pituitary 9:317–321
Ferrante E, Ferraroni M, Castrignanò T, Menicatti L, Agnani M, Reimondo G, Del Monte P, Bernasconi D, Loli P, Faustini-Fustini M, Borretta G, Terzolo M, Losa M, Morabito A, Spada A, Beck-Peccoz P, Lania AG (2006) Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumours. Eur J Endocrinol 155:823–829
Turner HE, Stratton IM, Byrne JV, Adams CBT, Wass JAH (1999) Audit of selected patients with nonfunctioning pituitary adenomas treated without irradiation-a follow-up study. Clin Endocrinol 51:281–284
Colao A, Gerbone G, Cappabianca P, Ferone D, Alfieri A, Di Salle F, Faggiano A, Merola B, de Divitiis E, Lombardi G (1998) Effect of surgery and radiotherapy in visual and endocrine function in nonfunctioning pituitary adenomas. J Endocrinol Invest 21:284–290
Boelaert K, Gittoes N (2001) Radiotherapy for non functioning pituitary adenomas. Eur J Endocrinol 144:569–575
De Martino MC, Hofland LJ, Lamberts SWJ (2010) Somatostatin and somatostatin receptors: from basic concepts to clinical applications. Prog Brain Res 182:255–280
de Herder WW, Kwekkeboom DJ, Feelders RA, van Aken MO, Lamberts SW, van der Lely AJ, Krenning EP (2006) Somatostatin receptor imaging for neuroendocrine tumors. Pituitary 9:243–248
Taboada GF, Luque RM, Bastos W, Guimaraes R, Marcondes J, Chimelli L, Fontes L, Mata P, Filho P, Carvalho D, Kineman R, Gadelha M (2007) Quantitative analysis of somatostatin receptors subtype (SSTR1–5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol 156:65–74
Greenman Y, Melmed S (1994) Heterogeneous expression of two somatostatin receptor subtypes in pituitary tumors. J Clin Endocrinol Metab 78:398–403
Greenman Y, Melmed S (1994) Expression of three somatostatin receptor subtypes in pituitary adenomas: evidence for preferential SSTR5 expression in the mammosomatotroph lineage. J Clin Endocrinol Metab 79:724–729
Miller G, Alexander J, Bikkal H, Katznelson L, Zervas N, Klibanski A (1995) Somatostatin receptor subtype gene expression in pituitary adenomas. J Clin Endocrinol Metab 80:1386–1392
Pawlikowski M, Pisarek H, Kunert-Radek J, Radek A (2003) Immunohistochemical detection of somatostatin receptor subtypes in “clinically nonfunctioning pituitary adenomas”. Endocr Pathol 14:231–238
Ben-Shlomo A, Melmed S (2010) Pituitary somatostatin receptor signaling. Trends Endocrinol Metab 21(3):123–133
Ben-Shlomo A, Wawrowsky KA, Proekt I, Wolkenfeld NM, Ren S-G, Taylor J, Culler MD, Melmed S (2005) Somatostatin receptor type 5 modulates somatostatin receptor type 2 regulation of adrenocorticotropin secretion. J Biol Chem 280(25):24011–24021
Pawlikowski M, Lawnicka H, Pisarek H, Kunert-Radek J, Radek M, Culler MD (2007) Effects of somatostatin-14 and the receptor-specific somatostatin analogs on chromogranin A and alpha-subunit (alpha-SU) release from “clinically nonfunctioning” pituitary adenoma cells incubated in vitro. J Physiol Pharmacol 58:179–188
Florio T, Thellung S, Arena S, Corsaro A, Spaziante R, Gussoni G, Acuto G, Giusti M, Giordano G, Schettini G (1999) Somatostatin and its analog lanreotide inhibit the proliferation of dispersed human non-functioning pituitary adenoma cells in vitro. Eur J Endocrinol 141:396–408
Gruszka A, Kunert-Radek J, Radek A, PisareK H, Taylor J, Dong JZ, Culler MD, Pawlikowski M (2006) The effect of selective sst1, sst2 sst5 somatostatin receptors agonists, a somatostatin/dopamine (SST/DA) chimera and bromocriptine on the “clinically non functioning” pituitary adenomas in vitro. Life Sciences 78:689–693
Padova H, Rubinfeld H, Hadani M, Cohen ZR, Nass D, Taylor JE, Culler MD, Shimon I (2008) Effects of selective somatostatin analogs and cortistatin on cell viability in cultured human non-functioning pituitary adenomas. Mol Cell Endocrinol 286:214–218
Zatelli MC, Piccin D, Vignali C, Tagliati F, Ambrosio MR, Bondanelli M, Cimino V, Bianchi A, Schmid HA, Scanarini M, Pontecorvi A, De Marinis L, Maira G, Degli Uberti EC (2007) Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non-functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr Rel Cancer 14:91–102
De Bruin T, Kwekkeboom D, Van’t Verlaat J, Reubi JC, Krenning E, Lamberts S, Croughs R (1992) Clinically nonfunctioning pituitary adenoma and octreotide response to long term high dose treatment and studies in vitro. J Clin Endocrinol Metab 75:1310–1317
Gasperi M, Petrini L, Pilosu R, Nardi M, Marcello A, Mastio F, Bartalena L, Martino E (1993) Octreotide treatment does not affect the size of most non-functioning pituitary adenomas. J Endocrinol Invest 16:541–543
Borson-Chazot F, Houzard C, Ajzenberg C, Nocaudie M, Duet M, Mundler O, Marchandise X, Epelbaum J, Gomez De Alzaga M, Schafer J, Meyerhof W, Sassolas G, Warnet A (1997) Somatostatin receptor imaging in somatotroph and non-functioning pituitary adenomas: correlation with hormonal and visual responses to octreotide. Clin Endocrinol (Oxf) 47:589–598
Merola B, Colao A, Ferone D, Selleri A, Di Sarno A, Marzullo P, Biondi B, Spaziante R, Rossi E, Lombardi G (1993) Effects of a chronic treatment with octreotide in patients with functionless pituitary adenomas. Horm Res 40:149–155
Warnet A, Harris AG, Renard E, Martin D, James-Deidier A, Chaumet-Riffaud P, the French multicenter octreotide study group (1997) A prospective multicenter trial of octreotide in 24 patients with visual defects caused by nonfunctioning and gonadotropin secreting pituitary adenomas. Neurosurgery 41:786–797
Plockinger U, Reichel M, Fett U, Saeger W, Quabbe HJ (1994) Preoperative octreotide treatment of growth-hormone secreting and clinically nonfunctioning pituitary macroadenomas: effect on tumor volume and lack of correlation with immunohistochemistry and somatostatin receptor scintigraphy. J Clin Endocrinol Metab 79:1416–1423
Colao A, Lastoria S, Ferone D, Varrella P, Marzullo P, Pivonello R, Cerbone G, Acampa W, Salvatore M, Lombardi G (1999) Pituitary uptake of In-111-DTPA-D-Phe-octreotide in the normal pituitary and in pituitary adenomas. J Endocrinol Invest 22:176–183
Feldkamp J, Santen R, Harms E, Aulich A, Modder U, Scherbaum WA (1999) Incidentally discovered pituitary lesions: high frequency of macroadenomas and hormone-secreting adenomas-results of a prospective study. Clin Endocrinol (Oxf) 51:109–113
Dekkers OM, Hammer S, de Keizer RJW, Roelfsema F, Schutte PJ, Smit JWA, Romijn JA, Pereira AM (2007) The natural course of non-functioning pituitary macroadenomas. Eur J Endocrinol 156:217–224
Wass JA, Karavitaki N (2009) Nonfunctioning pituitary adenomas: the Oxford experience. Nat Rev Endocrinol 5:519–522
Greenman Y, Ouaknine G, Veshchev I, Reider-Groswasser I, Segev Y, Stern N (2003) Postoperative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumour quiescence and regrowth. Clin Endocrinol 58:763–769
Acknowledgments
Authors wish to acknowledge Paola Lanza (Institute of Pathology, Catholic University, Rome, Italy) who contributed to immunohistochemistry.
Conflict of interest
The Authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fusco, A., Giampietro, A., Bianchi, A. et al. Treatment with octreotide LAR in clinically non-functioning pituitary adenoma: results from a case–control study. Pituitary 15, 571–578 (2012). https://doi.org/10.1007/s11102-011-0370-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-011-0370-8