Abstract
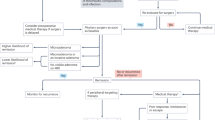
The majority of pituitary tumors that cause Cushing's disease are small (<1 cm diameter), and most disease morbidity is due to the effects of elevated, non-suppressible, ACTH levels that these tumors secrete. Tumor-derived ACTH leads to adrenal-derived steroid hypersecretion and results in many disabling and sometimes life-threatening symptoms including abnormal fat deposition, skin thinning, psychological disturbances, hypertension, diabetes, osteoporosis and muscle weakness. Cushing's disease is associated with high morbidity and ultimately mortality. In experienced specialized centers, 70% of corticotroph microadenomas can be successfully resected by transsphenoidal pituitary surgery. However, surgical “cure” rates for larger ACTH-secreting pituitary tumors are achieved in only 30% of cases, and recent reports highlight a significant recurrence rate after longer term follow-up even in smaller tumors. Post-surgical persistence of ACTH hypersecretion may require pituitary-directed radiation, but this treatment may take some time to be effective, and like extensive surgical pituitary tumor resection, ultimately leads to partial- or total hypopituitarism in ∼80% of cases. Although hypercortisolism may be completely resolved by adrenalectomy, this procedure does not suppress, and may act as a stimulus to pituitary tumor growth, and is associated with other co-morbidity. Although some currently available drug-based treatments for Cushing's disease effectively control hypercortisolism, their drawback has been that they do not impact on pituitary tumor growth. Recent studies have identified the potential utility of peroxisome-proliferator activating receptor-gamma (PPAR-γ) novel ligands in in vitro, and in vivo Cushing's disease models, and have paved the way for early clinical studies to develop novel therapeutic approaches in Cushing's disease.
Similar content being viewed by others
References
Merrill RM, Feuer EJ, Capocaccia R, Mariotto A. Cancer prevalence estimates based on tumor registry data in the SEER program. Int J Epidemiol 2000;29:197–207.
Central Brain Tumor Registry of the United States (CBTRUS). http://www.cbtrus.org.
Jukich PJ, McCarthy BJ, Surawicz TS, Freels S, Davis FG. Trends in incidence of primary brain tumors in the United States, 1985–1994. Neuro-Oncology 2001;3:141–151.
Freda PU, Wardlaw SL. Diagnosis and treatment of pituitary tumors. J Clin Endocrinol Metab 1999;84:3859–3866.
Giustina A, Barkan A, Casanueva FF, et al. Criteria for cure of acromegaly: a consensus statement. J Clin Endocrinol Metab 2000;85:526–529.
Niemann LK. Medical Therapy of Cushing's Disease. Pituitary 2002;5:77–82.
Morris D, Grossman A. The Medical Management of Cushing's Syndrome. Ann NY Acad Sci 2002;970:119–133.
Heaney AP, Melmed S. Pituitary Tumors: Molecular Targets and New Medical Therapies. Nature Reviews Cancer 2004;4:285–295.
Orth DN. Cushing's syndrome. N Engl J Med 1995;332:791–803.
Simmons NE, Alden TD, Thorner MO, Laws ER Jr. Serum cortisol response to transphenoidal surgery for Cushing disease. J Neurosurg 2001;95:1–8.
Mampalam TJ, Tyrrell JB, Wilson CB. Transsphenoidal microsurgery for Cushing's disease: A report of 216 cases. Ann Intern Med 1988;109:487–493.
Barbetta L, Dall'Asta C, Tomei G, Locatelli M, Giovanelli M, Ambrosi B. Assessment of cure and recurrence after pituitary surgery for Cushing's disease. Acta Neurochirurgica (Wien) 2001;143:477–481.
Rees DA, Hanna FW, Davies JS, Miles RG, Vafidis J, Scanlon MF. Long-term follow-up results of transsphenoidal surgery for Cushing's disease in a single center using strict criteria for remission. Clin Endocrinol 2002;56:541–551.
Verlhest JA, Trainer PJ, Howlett TA, et al. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing's syndrome. Clin Endocrinol 1991;35:169–178.
Tabarin A, Navarranne A, Guerin J, Corcuff JB, Parmiex M, Roger P. Use of ketaconazole in the treatment of Cushing's diaease and ectopic ACTH syndrome. Clin Endocrinol 1991;34:63–69.
Sonino N, Boscaro M, Merola G, Mantero F. Prolonged treatment of Cushing's disease by ketoconazole. J Clin Endocrinol Metab 1985;61:718–722.
McCance DR, Ritchie CM, Sheridan H, Atkinson AB. Acute hypoadrenalism and hepatotoxicity after treatment with ketoconazole. Lancet 1981;1:573.
Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 1990;347:645–660.
Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signaling pathways through heterodimer formation of their receptors. Nature 1992;358:771–774.
Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes 1998;47:507–514.
Heaney AP, Fernando M, Yong W, Melmed S. Functional PPAR-γ receptor represents a novel therapeutic target in Cushing's disease. Nat Med 2002;11:1281–1287.
Heaney AP, Fernando M, Melmed S. PPAR-γ receptor ligands: A novel therapy for pituitary tumors. J Clin Invest 2003;111:1381–1388.
Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 1996;45:1661–1669.
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 1998;391:79–82.
Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPAR gamma. Nat Med 1998;4:1046–1052.
Koeffler HP. Peroxisome proliferator-activated receptor γ and cancers. Clin Cancer Res 2003;9:1–9.
Gruszka A, Kunert-Radek J, Pawlikowski M. Rosiglitazone, PPAR-gamma ligand decreases the viability of rat prolactin-secreting pituitary tumor cells in vitro. Neuro Endocrinol Lett 2005;26:51–54.
Bogazzi F, Ultimieri F, Raggi F, Russo D, Vanacore R, Guida C, Viacava P, Cecchetti D, Acerbi G, Brogioni S, Cosci C, Gasperi M, Bartalena L, Martino E. PPAR-γ inhibits GH synthesis and secretion and increases apoptosis of pituitary GH-secreting adenomas. Eur J Endocrinol 2004;150:863–875.
Alevizaki M, Philippou G, Zapanti L, Alevizaki CC, Anastasiou E, and Mavrikakis M. Significant improvement of recurrent pituitary-dependent Cushing's syndrome after administration of a PPAR-gamma agonist. The Endocrine Society's 86th Annual Meeting, New Orleans, 2004. P2-453 (abstract).
Hull SSA, Sheridan B, Atkinson AB. Pre-operative medical therapy with rosiglitazone in two patients with newly diagnosed pituitary-dependent Cushing's syndrome. Clin Endocrinol 2004;62:258–262.
Heaney AP, Drange MR, Melmed S. Functional PPAR- receptor is a novel target for ACTH-secreting pituitary adenomas (Cushing's disease). The Endocrine Society's 85th Annual Meeting, Philadelphia 2003. OR7-4 (abstract).
Ambrosi B, Dall'Asta C, Cannavo S, Libe R, Vigo T, Epaminonda P, Chiodini I, Ferrero S, Trimarchi F, Arosio M, Beck-Pecoz P. Effects of chronic administration of PPAR-γ receptor ligand rosiglitazone in Cushing's disease. Eur J Endocrinol 2004;151:1–7.
Zhu Y, Fernando M, Heaney AP. PPAR-γ binds a putative PPRE in the POMC promoter. The Endocrine Society's 86th Annual Meeting, New Orleans 2004. OR33-6 (abstract).
Suri D, Weiss RE. Effect of Pioglitazone on ACTH and cortisol secretion in Cushing's disease. J Clin Endocrinol Metab 2005;90:1340–1346.
Boyle PJ, King AB, Olansky L, Marchetti A, Lau H, Magar R, Martin J. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Clin Therap 2002;24:378–396.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heaney, A.P. PPAR-γ in Cushing's Disease. Pituitary 7, 265–269 (2004). https://doi.org/10.1007/s11102-005-1430-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-005-1430-8