Abstract
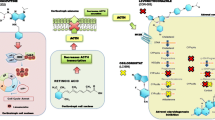
Somatostatin (SRIF) has been proposed to be of therapeutic interest in the medical treatment of Cushing's disease. While in vitro data demonstrate the presence of SRIF-receptor subtype (sst) expression in corticotroph adenomas, the current clinically available SRIF-analog Octreotide, predominantly targeting sst2, is ineffective in lowering ACTH levels in Cushing's disease and only appears to inhibit the release of ACTH in Nelson's syndrome. In the present review, current knowledge on the physiological role of SRIF in the regulation of ACTH secretion by the anterior pituitary gland, as well as by corticotroph tumor cells is summarized. In addition, the role of glucocorticoids in regulating sst-mediated inhibition of ACTH release by corticotroph adenoma cells is discussed. Recently, it was reported that the novel multiligand SRIF-analog SOM230 might have the potential to be of therapeutic interest for Cushing's disease. On the basis of the potent suppressive effects on ACTH release by SRIF-analogs with high binding affinity to sst5 and the observation that sst5 expression and action is relatively resistant to glucocorticoid treatment, including the recent observation that sst5 is the predominant sst expressed in human corticotroph adenomas, it is hypothesized that sst5 may be a new therapeutic target for the control of ACTH and cortisol hypersecretion in patients with pituitary dependent Cushing's disease.
Similar content being viewed by others
References
Cushing H. The basophil adenomas of the pituitary body and their clinical manifestations. Bull Johns Hopkins Hosp 1932;137–195.
Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endocr Rev 1998;5:647–672.
Mampalam TJ, Tyrrell JB, Wilson CB. Transsphenoidal microsurgery for Cushing disease. A report of 216 cases. Ann Intern Med 1988;6:487–493.
Swearingen B, Biller BM, Barker FG, 2nd, Katznelson L, Grinspoon S, Klibanski A, Zervas NT. Long-term mortality after transsphenoidal surgery for Cushing disease. Ann Intern Med 1999;10:821–824.
Trainer PJ, Lawrie HS, Verhelst J, Howlett TA, Lowe DG, Grossman AB, Savage MO, Afshar F, Besser GM. Transsphenoidal resection in Cushing's disease: Undetectable serum cortisol as the definition of successful treatment. Clin Endocrinol (Oxf) 1993;1:73–78.
Knappe UJ, Ludecke DK. Persistent and recurrent hypercortisolism after transsphenoidal surgery for Cushing's disease. Acta Neurochir Suppl 1996;31–34.
Boggan JE, Tyrrell JB, Wilson CB. Transsphenoidal microsurgical management of Cushing's disease. Report of 100 cases. J Neurosurg 1983;2:195–200.
Sonino N, Zielezny M, Fava GA, Fallo F, Boscaro M. Risk factors and long-term outcome in pituitary-dependent Cushing's disease. J Clin Endocrinol Metab 1996;7:2647–2652.
Colao A, Di Sarno A, Marzullo P, Di Somma C, Cerbone G, Landi ML, Faggiano A, Merola B, Lombardi G. New medical approaches in pituitary adenomas. Horm Res 2000;76–87.
Miller JW, Crapo L. The medical treatment of Cushing's syndrome. Endocr Rev 1993;4:443–458.
Morris D, Grossman A. The medical management of Cushing's syndrome. Ann N Y Acad Sci 2002;119–133.
Orrego JJ, Barkan AL. Pituitary disorders. Drug treatment options. Drugs 2000;1:93–106.
Yap LB, Turner HE, Adams CB, Wass JA. Undetectable postoperative cortisol does not always predict long-term remission in Cushing's disease: A single centre audit. Clin Endocrinol (Oxf) 2002;1:25–31.
Estrada J, Garcia-Uria J, Lamas C, Alfaro J, Lucas T, Diez S, Salto L, Barcelo B. The complete normalization of the adrenocortical function as the criterion of cure after transsphenoidal surgery for Cushing's disease. J Clin Endocrinol Metab 2001;12:5695–5699.
Chee GH, Mathias DB, James RA, Kendall-Taylor P. Transsphenoidal pituitary surgery in Cushing's disease: Can we predict outcome? Clin Endocrinol (Oxf) 2001;5:617–626.
Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF. Long-term follow-up results of transsphenoidal surgery for Cushing's disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf) 2002;4:541–551.
Pereira AM, van Aken MO, van Dulken H, Schutte PJ, Biermasz NR, Smit JW, Roelfsema F, Romijn JA. Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing's disease. J Clin Endocrinol Metab 2003;12:5858–5864.
Tyrrell JB, Lorenzi M, Gerich JE, Forsham PH. Inhibition by somatostatin of ACTH secretion in Nelson's syndrome. J Clin Endocrinol Metab 1975;6:1125–1127.
Kemink SA, Grotenhuis JA, De Vries J, Pieters GF, Hermus AR, Smals AG. Management of Nelson's syndrome: Observations in fifteen patients. Clin Endocrinol (Oxf) 2001;1:45–52.
Lamberts SW, Uitterlinden P, Klijn JM. The effect of the long-acting somatostatin analogue SMS 201-995 on ACTH secretion in Nelson's syndrome and Cushing's disease. Acta Endocrinol (Copenh) 1989;6:760–766.
Stalla GK, Brockmeier SJ, Renner U, Newton C, Buchfelder M, Stalla J, Muller OA. Octreotide exerts different effects in vivo and in vitro in Cushing's disease. Eur J Endocrinol 1994;2:125–131.
Ambrosi B, Bochicchio D, Fadin C, Colombo P, Faglia G. Failure of somatostatin and octreotide to acutely affect the hypothalamic-pituitary-adrenal function in patients with corticotropin hypersecretion. J Endocrinol Invest 1990;3:257–261.
Lewis I, Bauer W, Albert R, Chandramouli N, Pless J, Weckbecker G, Bruns C. A novel somatostatin mimic with broad somatotropin release inhibitory factor receptor binding and superior therapeutic potential. J Med Chem 2003;12:2334–2344.
Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973;68:77–79.
Freda PU. Somatostatin analogs in acromegaly. J Clin Endocrinol Metab 2002;7:3013–3018.
Kleinberg DL. Primary therapy for acromegaly with somatostatin analogs and a discussion of novel Peptide analogs. Rev Endocr Metab Disord 2005;1:29–37.
Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: Biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2003;12:999–1017.
O'Carroll AM, Krempels K. Widespread distribution of somatostatin receptor messenger ribonucleic acids in rat pituitary. Endocrinology 1995;11:5224–5227.
Day R, Dong W, Panetta R, Kraicer J, Greenwood MT, Patel YC. Expression of mRNA for somatostatin receptor (sstr) types 2 and 5 in individual rat pituitary cells. A double labeling in situ hybridization analysis. Endocrinology 1995;11:5232–5235.
Mezey E, Hunyady B, Mitra S, Hayes E, Liu Q, Schaeffer J, Schonbrunn A. Cell specific expression of the sst2A and sst5 somatostatin receptors in the rat anterior pituitary. Endocrinology 1998;1:414–419.
Shimon I, Taylor JE, Dong JZ, Bitonte RA, Kim S, Morgan B, Coy DH, Culler MD, Melmed S. Somatostatin receptor subtype specificity in human fetal pituitary cultures. Differential role of SSTR2 and SSTR5 for growth hormone, thyroid-stimulating hormone, and prolactin regulation. J Clin Invest 1997;4:789–798.
Brown MR, Rivier C, Vale W. Central nervous system regulation of adrenocorticotropin secretion: Role of somatostatins. Endocrinology 1984;5:1546–1549.
Kraicer J, Gajewski TC, Moor BC. Release of pro-opiomelanocortin-derived peptides from the pars intermedia and pars distalis of the rat pituitary: Effect of corticotrophin-releasing factor and somatostatin. Neuroendocrinology 1985;5:363–373.
Voight KH, Fehm HL, Lang RE, Walter R. The effect of somatostatin and of prolyl-leucyl-glycinamide (MIF) on ACTH release in dispersed pituitary cells. Life Sci 1977;5:739–745.
Nicholson SA, Adrian TE, Gillham B, Jones MT, Bloom SR. Effect of hypothalamic neuropeptides on corticotrophin release from quarters of rat anterior pituitary gland in vitro. J Endocrinol 1984;2:219–226.
Lamberts SW, Zuyderwijk J, den Holder F, van Koetsveld P, Hofland L. Studies on the conditions determining the inhibitory effect of somatostatin on adrenocorticotropin, prolactin and thyrotropin release by cultured rat pituitary cells. Neuroendocrinology 1989;1:44–50.
Lamberts SW. The role of somatostatin in the regulation of anterior pituitary hormone secretion and the use of its analogs in the treatment of human pituitary tumors. Endocr Rev 1988;4:417–436.
Invitti C, Pecori Giraldi F, Dubini A, Piolini M, Cavagnini F. Effect of sandostatin on CRF-stimulated secretion of ACTH, beta-lipotropin and beta-endorphin. Horm Metab Res 1991;5:233–235.
Stafford PJ, Kopelman PG, Davidson K, McLoughlin L, White A, Rees LH, Besser GM, Coy DH, Grossman A. The pituitary-adrenal response to CRF-41 is unaltered by intravenous somatostatin in normal subjects. Clin Endocrinol (Oxf) 1989;6:661–666.
Benker G, Hackenberg K, Hamburger B, Reinwein D. Effects of growth hormone release-inhibiting hormone and bromocryptine (CB 154) in states of abnormal pituitary-adrenal function. Clin Endocrinol (Oxf) 1976;2:187–190.
Lamberts SW, den Holder F, Hofland LJ. The interrelationship between the effects of insulin-like growth factor I and somatostatin on growth hormone secretion by normal rat pituitary cells: The role of glucocorticoids. Endocrinology 1989;2:905–911.
Djordjijevic D, Zhang J, Priam M, Viollet C, Gourdji D, Kordon C, Epelbaum J. Effect of 17beta-estradiol on somatostatin receptor expression and inhibitory effects on growth hormone and prolactin release in rat pituitary cell cultures. Endocrinology 1998;5:2272–2277.
Visser-Wisselaar HA, Van Uffelen CJ, Van Koetsveld PM, Lichtenauer-Kaligis EG, Waaijers AM, Uitterlinden P, Mooy DM, Lamberts SW, Hofland LJ. 17-beta-estradiol-dependent regulation of somatostatin receptor subtype expression in the 7315b prolactin secreting rat pituitary tumor in vitro and in vivo. Endocrinology 1997;3:1180–1189.
Fehm HL, Voigt KH, Lang R, Beinert KE, Raptis S, Pfeiffer EF. Somatostatin: A potent inhibitor of ACTH-hypersecretion in adrenal insufficiency. Klin Wochenschr 1976;4:173–175.
Julesz J, Laczi F, Janaky T, Laszlo F. Effects of somatostatin and bromocryptine on the plasma ACTH level in bilaterally adrenalectomized patients with Cushing's disease. Endokrinologie 1980;1:68–72.
Kelestimur F, Utas C, Ozbakir O, Selcuklu A, Kandemir O, Ozcan N. The effects of octreotide in a patient with Nelson's syndrome. Postgrad Med J 1996;843:53–54.
Petrini L, Gasperi M, Pilosu R, Marcello A, Martino E. Long-term treatment of Nelson's syndrome by octreotide: A case report. J Endocrinol Invest 1994;2:135–139.
De Herder WW, Lamberts SW. Octapeptide somatostatin-analogue therapy of Cushing's syndrome. Postgrad Med J 1999;880:65–66.
Lamberts SW, Hofland LJ, de Herder WW, Kwekkeboom DJ, Reubi JC, Krenning EP. Octreotide and related somatostatin analogs in the diagnosis and treatment of pituitary disease and somatostatin receptor scintigraphy. Front Neuroendocrinol 1993;1:27–55.
De Herder WW, Kwekkeboom DJ, Reijs AEM, Kooy PPM, Hofland LJ, Krenning EP, Lamberts SWJ. Receptor scintigraphy with somatostatin analogues and dopamine antagonists of pituitary tumours. In K. von Werder and R. Fahlbusch. Pituitary adenomas. From basic research to diagnosis and therapy. Amsterdam, Elsevier Science BV. 1996:93–104.
Schonbrunn A. Glucocorticoids down-regulate somatostatin receptors on pituitary cells in culture. Endocrinology 1982;4:1147–1154.
Richardson UI, Schonbrunn A. Inhibition of adrenocorticotropin secretion by somatostatin in pituitary cells in culture. Endocrinology 1981;1:281–290.
Tallent M, Liapakis G, O'Carroll AM, Lolait SJ, Dichter M, Reisine T. Somatostatin receptor subtypes SSTR2 and SSTR5 couple negatively to an L-type Ca2+ current in the pituitary cell line AtT-20. Neuroscience 1996;4:1073–1081.
Strowski MZ, Dashkevicz MP, Parmar RM, Wilkinson H, Kohler M, Schaeffer JM, Blake AD. Somatostatin receptor subtypes 2 and 5 inhibit corticotropin-releasing hormone-stimulated adrenocorticotropin secretion from AtT-20 cells. Neuroendocrinology 2002;6:339–346.
Cervia D, Fehlmann D, Hoyer D. Native somatostatin sst2 and sst5 receptors functionally coupled to G(i/o)-protein, but not to the serum response element in AtT-20 mouse tumour corticotrophs. Naunyn Schmiedebergs Arch Pharmacol 2003;6:578–587.
Cervia D, Nunn C, Fehlmann D, Langenegger D, Schuepbach E, Hoyer D. Pharmacological characterisation of native somatostatin receptors in AtT-20 mouse tumour corticotrophs. Br J Pharmacol 2003;1:109–121.
Ben-Shlomo A, Miklovsky I, Wawrosky K, Ren S, Taylor J, Culler M, Melmed S. Visualization and function of pituitary receptor subtypes in live ACTH-secreting AtT20 cells. The Endocrine Society Meeting 2004;Abstract P3–244.
Miller GM, Alexander JM, Bikkal HA, Katznelson L, Zervas NT, Klibanski A. Somatostatin receptor subtype gene expression in pituitary adenomas. J Clin Endocrinol Metab 1995;4:1386–1392.
Panetta R, Patel YC. Expression of mRNA for all five human somatostatin receptors (hSSTR1-5) in pituitary tumors. Life Sci 1995;5:333–342.
Greenman Y, Melmed S. Heterogeneous expression of two somatostatin receptor subtypes in pituitary tumors. J Clin Endocrinol Metab 1994;2:398–403.
Nielsen S, Mellemkjaer S, Rasmussen LM, Ledet T, Olsen N, Bojsen-Moller M, Astrup J, Weeke J, Jorgensen JO. Expression of somatostatin receptors on human pituitary adenomas in vivo and ex vivo. J Endocrinol Invest 2001;6:430–437.
Hofland LJ, van der Hoek J, Feelders R, van Aken MO, van Koetsveld PM, Waaijers M, Sprij-Mooij D, Bruns C, Weckbecker G, de Herder WW, Beckers A, Lamberts SWJ. The multiligand somatostatin analog SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor subtype 5. Eur J Endocrinol 2005;152:645–654.
Weckbecker G, Briner U, Lewis I, Bruns C. SOM230: A new somatostatin peptidomimetic with potent inhibitory effects on the growth hormone/insulin-like growth factor-I axis in rats, primates, and dogs. Endocrinology 2002;10:4123–4130.
Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 2002;5:707–716.
van der Hoek J, de Herder WW, Feelders RA, van der Lely AJ, Uitterlinden P, Boerlin V, Bruns C, Poon KW, Lewis I, Weckbecker G, Krahnke T, Hofland LJ, Lamberts SW. A single-dose comparison of the acute effects between the new somatostatin analog SOM230 and octreotide in acromegalic patients. J Clin Endocrinol Metab 2004;2:638–645.
van der Hoek J, Waaijers A, van Koetsveld P, Sprij-Mooij D, Feelders RA, Schmid HA, P. S, Hoyer D, Cervia D, J.E. T, M.D. C, Lamberts SWJ, Hofland LJ. Distinct functional properties of native somatostatin receptor subtype 5 compared with subtype 2 in the regulation of ACTH release by corticotroph tumor cells. Am J Physiol Endocrinol Metab 2005; in press.
Patel YC, Panetta R, Escher E, Greenwood M, Srikant CB. Expression of multiple somatostatin receptor genes in AtT-20 cells. Evidence for a novel somatostatin-28 selective receptor subtype. J Biol Chem 1994;2:1506–1509.
Vanetti M, Kouba M, Wang X, Vogt G, Hollt V. Cloning and expression of a novel mouse somatostatin receptor (SSTR2B). FEBS Lett 1992;3:290–294.
George SR, Kovacs K, Asa SL, Horvath E, Cross EG, Burrow GN. Effect of SMS 201-995, a long-acting somatostatin analogue, on the secretion and morphology of a pituitary growth hormone cell adenoma. Clin Endocrinol (Oxf) 1987;4:395–405.
Kraus J, Woltje M, Schonwetter N, Hollt V. Alternative promoter usage and tissue specific expression of the mouse somatostatin receptor 2 gene. FEBS Lett 1998;3:165–170.
Kraus J, Woltje M, Hollt V. Regulation of mouse somatostatin receptor type 2 gene expression by glucocorticoids. FEBS Lett 1999;2:200–204.
Gordon DF, Woodmansee WW, Lewis SR, James RA, Wood WM, Ridgway EC. Cloning of the mouse somatostatin receptor subtype 5 gene: Promoter structure and function. Endocrinology 1999;12:5598–5608.
Xu Y, Berelowitz M, Bruno JF. Dexamethasone regulates somatostatin receptor subtype messenger ribonucleic acid expression in rat pituitary GH4C1 cells. Endocrinology 1995;11:5070–5075.
Park S, Kamegai J, Kineman RD. Role of glucocorticoids in the regulation of pituitary somatostatin receptor subtype (sst1-sst5) mRNA levels: Evidence for direct and somatostatin-mediated effects. Neuroendocrinology 2003;3:163–175.
Strowski MZ, Kohler M, Chen HY, Trumbauer ME, Li Z, Szalkowski D, Gopal-Truter S, Fisher JK, Schaeffer JM, Blake AD, Zhang BB, Wilkinson HA. Somatostatin receptor subtype 5 regulates insulin secretion and glucose homeostasis. Mol Endocrinol 2003;1:93–106.
Shimon I. Somatostatin receptors in pituitary and development of somatostatin receptor subtype-selective analogs. Endocrine 2003;3:265–270.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
der Hoek, J.v., Lamberts, S.W.J. & Hofland, L.J. The Role of Somatostatin Analogs in Cushing's Disease. Pituitary 7, 257–264 (2004). https://doi.org/10.1007/s11102-005-1404-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-005-1404-x