Abstract
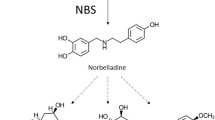
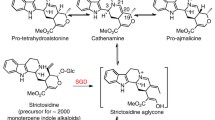
The biosynthetic pathway leading to the monoterpenoid indole alkaloid ajmaline in Rauvolfia serpentiin serpentina is one of the most studied in the field of natural product biosynthesis. Ajmaline has a complex structure which is based on a six-membered ring system harbouring nine chiral carbon atoms. There are about fifteen enzymes involved, including some involving the side reactions of the ajmaline biosynthetic pathway. All enzymes exhibit pronounced substrate specificity. In the recent years isolation and sequencing of their cDNAs has allowed a detailed sequence analysis and comparison with functionally related and occasionally un-related enzymes. Site-directed mutations of several of the ajmaline-synthesizing enzymes have been performed and their catalytic residues have been identified. Success with over-expression of the enzymes was an important step for their crystallization and structural analysis by X-ray crystallography. Crystals with sufficient resolution were obtained from the major enzymes of the pathway. Strictosidine synthase has a 3D-structure with a six-bladed β-propeller fold the first time such a fold found in the plant kingdom. Its ligand complexes with tryptamine and secologanin, as well as structure-based sequence alignment, indicate a possible evolutionary relationship to several primary sequence-unrelated structures with this fold. The structure of strictosidine glucosidase was determined and its structure has as a (β/α)8 barrel fold. Vinorine synthase provides the first 3D structure of a member of BAHD enzyme super-family. Raucaffricine glucosidase involved in a side-route of ajmaline biosynthesis has been crystallized. The ajmaline biosynthetic pathway is an outstanding example where many enzymes 3D-structure have been known and where there is a real potential for protein engineering to yield new alkaloid.
Similar content being viewed by others
References
Barleben L, Ma X, Koepke J, Peng G, Michel H, Stöckigt J (2005) Expression, purification, crystallization and preliminary X-ray analysis of strictosidine glucosidase, an enzyme initiating biosynthetic pathways to a unique diversity of indole alkaloid skeletons. Biochim Biophys Acta 1747:89–92
Bayer A, Ma X, Stöckigt J (2004) Acetyltransfer in natural product biosynthesis – functional cloning and molecular analysis of vinorine synthase. Bioorg Med Chem 12:2787–2795
Bracher D, Kutchan TM (1992) Strictosidine synthase from Rauvolfia serpentina: analysis of a gene involved in indole alkaloid biosynthesis. Arch Biochem Biophys 294:717–723
Buglino J, Onwueme KC, Ferreras JA, Quadri LEN, Lima CD (2004) Crystal Structure of PapA5, a Phthiocerol Dimycocerosyl Transferase from Mycobacterium tuberculosis. J Biol Chem 279:30634–30642
Chrzanowska M, Rozwadowska MD (2004) Asymmetric synthesis of isoquinoline alkaloids. Chem Rev 104:3341–3370
Cox ED, Cook JC (1995) The Pictet–Spengler condensation: a new direction for an old reaction. Chem Rev 95:1791–1842
Creasey WA (1994) Pharmacology, biochemistry and clinical applications of the monoterpenoid alkaloids. In: Saxton JE (ed) Monoterpenoid Indole Alkaloids,␣Supplement to part 4. John Wiley & Sons, Chichester, New York, Brisbane, Toronto, Singapore, pp␣715–753
D’Auria JC (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9:331–340
De-Eknamkul W, Ounaroon A, Tanahashi T, Kutchan T, Zenk MH (1997) Phytochemistry 45:477–484
De-Eknamkul W, Suttipanta N, Kutchan TM (2000) Purification and characterization of deacetylipecoside synthase from Alangium lamarckii. Thw. Phytochemistry 55:177–181
DeLucas LJ, Hamrick D, Cosenza L, Nagy L, McCombs D, Bray T, Chait A, Stoops B, Belgovskiy A, Wilson WW, Parham M, Chernov N (2005) Protein crystallization: virtual screening and optimization. Prog Biophys Mol Biol 88:285–309
DeWaal A, Meijer AH, Verpoorte R (1995) Strictosidine synthase from Catharanthus roseus: purification and characterization of multiple forms. Biochem J 306:571–580
Edwards TA, Wilkinson BD, Wharton RP, Aggarwal AK (2003) Model of the brain tumor-pumilio translation repressor complex. Genes Dev 17:2508–2513
Falbe J, Regitz M (1991) In: RÖMPP Chemie Lexikon, Georg Thieme Verlag Stuttgart-New York, Vol. 4, pp 34–37
Geerlings A, Ibanez MM, Memelink J, van Der Heijden R, Verpoorte R. (2000) Molecular cloning and analysis of strictosidine beta-d-glucosidase, an enzyme in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Biol Chem 275:3051–3056
Gerasimenko I, Sheludko Y, Ma X, Stöckigt J (2002) Heterologous expression of a Rauvolfia cDNA encoding strictosidine glucosidase, a biosynthetic key to over 2000 monoterpenoid indole alkaloids. Eur J Biochem 269:2204–2213
Gerasimenko I, Ma X, Sheludko Y, Mentele R, Lottspeich F, Stöckigt J (2004) Purification and partial amino acid sequences of the enzyme vinorine synthase involved in a crucial step of ajmaline biosynthesis. Bioorg Med Chem 12:2781–2786
Gerlt JA, Raushel FM (2003) Evolution of function in (β/α)8 barrel enzymes. Curr Opin Chem Biol 7:252–264
Gibbs MR, Moody PCE, Leslie AGW (1990) Crystal Structure of the ASP-199 asparagine mutant of chloramphenicol acetyltransferase to 2.35. Å resolution: structural consequences of disruption of a buried salt bridge. Biochemistry 29:11261–11265
Gilliland GL, Tung M, Blakeslee DM, Ladner J (1994) The biological macromolecule crystallization database, version 3.0: new features, data, and the NASA archive for protein crystal growth data. Acta Crystallogr D 50:408–413
Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, Dvir H, Ravelle RBG, McCarthy A, Toker L, Silman L, Sussman JL, Tawfik DS (2004) Structure and evolution of the serum paraoxonase family of detoxifying and antiatherosclerotic enzymes. Nat Struct Mol Biol 11:412–419
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Henrissat B, Bairoch A (1996) Updating the sequence-based classification of glycosyl hydrolases. J Biochem 316:695–696
Hoffmann L, Maury S, Martz F, Geoffry P, Legrand L (2003) Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J␣Biol Chem 278:95–103
Jawad Z, Paoli M (2002) Novel sequences propel familiar folds. Structure 10:447–454
Jeon H, Meng W, Takagi J, Eck MJ, Springer TA, Blacklow SC (2001) Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol 8:499–504
Koepke J, Ma X, Fritzsch G, Michel H, Stöckigt J (2005) Crystallization and preliminary X-ray analysis of strictosidine synthase and its complex with the substrate tryptamine. Acta Crystallogr Sect D 61:690–693
Krupka HI, Rupp B, Segelke BW, Lekin TP, Wright D, Wu H-C, Todd P, Azarani A (2002) The high-speed Hydra-Plus-One system for a automated high-throughput protein crystallography. Acta Crystallogr D 58:1523–1526
Kutchan TM, Hampp N, Lottspeich F, Beyreuther K, Zenk MH (1988) The cDNA clone for strictosidine synthase from Rauvolfia serpentina. DNA sequence determination and expression in Escherichia coli. FEBS Lett 237:40–44
Kutchan TM (1993) Strictosidine: from alkaloid to enzyme to gene. Phytochemistry 32:493–506
Ma X, Koepke J, Fritzsch G, Diem R, Kutchan TM, Michel H, Stöckigt J (2004a) Crystallization and preliminary X-ray Crystallographic Analysis of strictosidine synthase from Rauvolfia – the first member of a novel enzyme family. Biochim Biophys Acta 1702:121–124
Ma X, Panjikar S, Koepke J Loris E, Stöckigt J (2006) The structure of Rauvolfia serpentina stritosidine synthase is a novel six-bladed beta-propeller fold in plant proteins. The Plant Cell 18:907–920
Ma X, Koepke J, Bayer A, Linhard V, Fritzsch G, Zhang B, Michel H, Stöckigt J (2004b) Vinorine synthase from Rauvolfia: the first example of crystallization and preliminary X-ray diffraction analysis of an enzyme of the BAHD superfamily. Biochim Biophys Acta 1701:129–132
Ma X, Koepke J, Bayer A, Fritzsch G, Michel H, Stöckigt J (2005a) Crystallization and preliminary X-ray analysis of native and selenomethionyl vinorine synthase from Rauvolfia serpentina. Acta Crystallogr Sect D 61:694–696
Ma X, Koepke J, Panjikar S, Fritzsch G, Stöckigt J (2005b) Crystal structure of vinorine synthase, the first representative of the BAHD superfamily. J Biol Chem 280:13576–13583
Panjikar S, Parthasarathy V, Lamzin VS, Weiss MS, Tucker PA (2005) Auto-Rickshaw: an automated crystal structure determination platform as an efficient tool for the validation of an X-ray diffraction experiment. Acta Cryst D 61:449–457
Pfitzner A, Polz L, Stöckigt J (1986) Properties of Vinorine Synthase – the Rauvolfia Enzyme involved in the Formation of the Ajmaline Skeleton. Z Naturforsch 41c:103–114
Pfitzner M, Zenk MH (1989) Homogenous strictosidine synthase isoenzymes from cell suspension cultures of Catharanthus roseus. Planta Med 55:525–530
Pictet A, Spengler T (1911) Über die Bildung von Isochinolin-derivaten durch Übertragung von Methylal auf Phenyl-äthylamin, Phenyl-alanin und Tyrosin. Ber Dtsch Chem Ges 44:2030–2036
Pons T, Gomez R, Chinea G, Valencia A (2003) Beta-propellers: associated functions and their role in human diseases. Curr Med Chem 10:505–524
Ruppert M, Stöckigt J (1999) Strictosidine – the biosynthetic key to monoterpenoid indole alkaloids. In: Sir␣Derek Barton, Nakanishi K (eds) Comprehensive␣Natural Products Chemistry Vol 4. Elsevier, Amsterdam, Lausanne, New York, Oxford, Shannon, Singapore, Tokyo, pp 109–138
Ruppert M, Ma X, Stöckigt J (2005) Alkaloid biosynthesis in Rauvolfia–cDNA cloning of major enzymes of the ajmaline pathway. In: Knölker H-J (eds) Current Org Chem 9:1431–1444
Ruppert M, Panjikar S, Barleben L, Stöckigt J (2006) Heterologous expression, purification, crystallization and preliminary X-ray analysis of raucaffricine glucosidase, a plant enzyme specifically involved in Rauvolfia alkaloid biosynthesis. Acta Crystallogr F 62:257–260
Ruyter CM, Stöckigt J (1991) Enzymatic formation of raucaffricine, the major indol alkaloid of Rauwolfia serpentina cell-suspension cultures. Helv Chim Acta 74:1707–1712
Samanani N, Liscombe DK, Facchini PJ (2004) Molecular cloning and characterization of norcoclaurine synthase, an enzyme catalyzing the first committed step in benzylisoquinoline alkaloid biosynthesis. Plant J 40:302–313
Scharff EI, Koepke J, Fritzsch G, Lücke C, Rüterjans H (2001) Crystal structure of diisopropylfluorophosphatase from Loligo vulgaris. Structure 9:493–502
Schübel H, Stöckigt J, Feicht R, Simon H (1986) Partial purification and characterization of raucaffricine β-D-glucosidase from plant cell-suspension cultures␣of Rauwolfia serpentina. Helv Chim Acta 69:538–547
St-Pierre B, DeLuca V (2000) Evolution of acyltransferase genes: origin and diversification of the BAHD superfamily of acyltransferases involved in secondary metabolism. In: John RI, Romeo T, Varin L, DeLuca V (eds) Recent Advances in Phytochemistry, Evolution of Metabolic Pathways Vol 34. Elsevier Science, Oxford, pp 285–315
Suzuki H, Nakayama T, Nishino T (2003) Proposed mechanism and functional amino acid residues of malonyl-CoA: anthocyanin 5-O-glucoside-6”’-O-malonyltransferase from flowers of Salvia splendens, a member of the versatile plant acyltransferase family. Biochemistry 42:1764–1771
Treimer JE, Zenk MH (1979) Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation. Eur J Biochem 101:225–233
Van Duyne GD, Standaert RF, Karplus A, Schreiber SL, Clardy J (1993) Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and Rapamycin. J Mol Biol 229:105–124
Warzecha H, Obitz P, Stöckigt J (1999) Purification, partial amino acid sequence and structure of the product of raucaffricine-O-beta-d-glucosidase from plant cell cultures of Rauvolfia serpentina. Phytochemistry 50:1099–1109
Warzecha H, Gerasimenko I, Kutchan TM, Stöckigt J (2000) Molecular cloning and functional bacterial expression of a plant glucosidase specifically involved in alkaloid biosynthesis. Phytochemistry 54:657–666
Yamazaki Y, Sudo H, Yamazaki M, Aimi N, Saito K (2003) Camptothecin biosynthetic genes in hairy roots of Ophiorrhiza pumila: cloning, characterization and differential expression in tissues and by␣stress compounds. Plant Cell Physiol 44: 395–403
Acknowledgements
We are very thankful to Mrs. Doris Rohr of our research group for cultivation of all plant cell systems, to Prof. F. Lottspeich and his coworkers Mrs. I. Mathes and Mr. R. Mentele (Max-Planck-Institute of Biochemistry, Martinsried, Germany) for their enthusiastic help in sequencing pre-purified Rauvolfia enzymes and to Prof. H. Michel, Dr. G. Fritzsch and Dr. J. Koepke (Max-Planck-Institute of Biophysics, Frankfurt/Main, Germany) for introducing us to structural biology research.
Our research was also supported by the Deutsche Forschungsgemeinschaft (Bonn, Bad-Godesberg, Germany), Fonds der Chemischen Industrie (Frankfurt/Main, Germany) and the Bundesministerium für Bildung und Forschung (BMBF), Bonn, Germany). Support in the form of access to synchrotron facilities by the European Community (Research Infrastructure Action under the FP6 “Structuring the European Research Area Programme”, contact number RII3/CT/2004/5060008) is also␣acknowledged and we thank Dr. Paul Tucker (EMBL Hamburg, Germany) for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stöckigt, J., Panjikar, S., Ruppert, M. et al. The molecular architecture of major enzymes from ajmaline biosynthetic pathway. Phytochem Rev 6, 15–34 (2007). https://doi.org/10.1007/s11101-006-9016-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-006-9016-2