Abstract
7-day soil drought followed by 7-day rehydration was applied to potted German chamomile (Chamomilla recutita) plants at the beginning of their generative stage. Plants of a wild type (WT), plus two diploid (2n) and two tetraploid (4n) genotypes were studied, in order to examine the alterations in chlorophyll (Chl) and carotenoids (Car) contents, and chlorophyll fluorescence (CF) parameters during water shortage and rehydration. The fresh mass of the anthodia after the recovery was also studied.
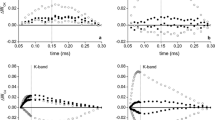
WT plants adjust better to water stress than modern breeding genotypes, because drought resulted in the low fall in leaf water content of WT, the lowest decrease in the fresh mass of its anthodia (a 41% decrease from the control), and the most elastic response of the photosynthetic apparatus. 4n C11/2 strain plants suffered from the highest reduction in anthodia yield (87%), and had the lowest constitutive pigment contents. It was also the only genotype which revealed nontypical alterations in various CF parameters obtained on a dark- and light-adapted leaf. During drought, a big increase was noticed in minimal, maximal, and variable fluorescence of PSII reaction centres in the dark- adapted (F0, Fm and Fv, respectively), and in the light-adapted state (F0′, Fm′ and Fv′). It was accompanied by the biggest decline in linear electron transport rate (ETR), quantum efficiency of PSII electron transport (ΦPSII) and photochemical quenching coefficient (qP). These alterations were prolonged to the stage when the normal leaf water content was retained. On the contrary, C6/2 strain plants had the highest constitutive Chl and Car contents, which additionally increased after rehydration, similarly to the values of F0, Fm and Fv, which reflects the high photosynthetic potential of this genotype. It was accompanied by the relatively high yield of its anthodia after drought. Considering the drop in the yield triggered by drought, it seems to be the only parameter which may be linked with the ploidy level.
Although the yield formation of chamomile strains cannot simply be estimated by CF assay, this technique may serve as an additional tool in the selection of plants to drought. The following circumstances should be submitted; namely: measurement at the proper developmental stage of plants, in different water regimes, and an analysis of various CF parameters. The increase in F0 and F0′, and the reduction in ETR, Fv′/Fm′, ΦPSII and qP values in response to water deficit should be an indicator of the impairment of the photosynthetic apparatus through drought.
Similar content being viewed by others
Abbreviations
- Car:
-
carotenoid
- CF:
-
chlorophyll fluorescence
- Chl:
-
chlorophyll
- F0, Fm :
-
minimal and maximal fluorescence in the dark-adapted state, respectively
- Fv :
-
variable fluorescence in the dark-adapted state
- Fv/Fm :
-
photochemical efficiency of PSII in the dark-adapted state
- F0′, Fm′:
-
minimal and maximal fluorescence in the light-adapted state, respectively
- Fv′:
-
variable fluorescence in the light-adapted state
- Fv′/Fm′:
-
PSII maximum efficiency
- ETR:
-
linear electron transport rate
- ΦPSII :
-
quantum efficiency of PSII electron transport
- NPQ:
-
nonphotochemical quenching of maximal CF
- PSII:
-
photosystem II
- qP :
-
photochemical quenching coefficient
References
Baker, N.R., Rosenqvist, E.: Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. — J. Exp. Bot. 55: 1607–1621, 2004.
Bączek-Kwinta, R., Adamska, A., Seidler-Łożykowska, K., Tokarz, K.: Does the rate of German chamomile growth and development influence the response of plants to soil drought? — Biologia 65: 837–842, 2010.
Bączek-Kwinta, R., Adamska, A., Seidler-Łożykowska, K.: [Growth patterns of aerial part sof some German chamomile genotypes as influenced by soil drought.] — Folia Hortic. 1: 54–60, 2006a. [In Polish.]
Bączek-Kwinta, R., Filek, W., Grzesiak, S., Hura, T. The effect of severe soil drought and rehydration on growth and antioxidative activity in flag leaves of winter triticale. — Biol. Plant.- 50: 55–60, 2006b.
Bączek-Kwinta, R., Seidler-Łożykowska, K.: Cultivars of German chamomile (Chamomilla recutita (L.) Rausch.) and their resistance to water stress. — Acta Physiol. Plant. 26: 142–143, 2004.
Bączek-Kwinta R., Kozieł A. [Reaction of photosynthetic apparatus of leaves and the field of heads of German chamomile subjected to drought.] — Advances Agr. Sci. 545: 103–116, 2010. [In Pol.]
Bernier, G., Havelange, A., Houssa, C., Petitjean, A., Lejeune, P.: Physiological signals that induce flowering. — Plant Cell 5: 1147–1155, 1993.
Bradford, K.J., Hsiao T.C.: Physiological responses to moderate water stress. — In: Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H. (ed).: Physiological Plant Ecology II. Water Relations and Carbon Assimilation. Pp. 263–324. Springer-Verlag Berlin — Heidelberg — New York 1982.
Bertamini, M., Nedunchezhian, N.: Photoinhibition of photosynthesis in mature and young leaves of grapevine (Vitis vinifera L.). — Plant Sci. 164: 635–644, 2003.
Cornic, G.: Drought stress inhibits photosynthesis by decreasing stomatal aperture, not by affecting ATP synthesis. — Trends Plant Sci. 5: 187–188, 2000.
Cornic, G., Le Gouallec, J.L., Briantais, J.M., Hodges, M.: Effect of dehydration and high light on photosynthesis of two C3 plants (Phaseolus vulgaris L. and Elatostema repens (Lour.) Hall f.). — Planta 177: 84–90, 1989.
Critchley, C.: Photoinhibition. — In: Raghavendra, A.S. (ed.): Photosynthesis: a Comprehensive Treatise. Pp. 264–272. Cambridge University Press, Cambridge 2000.
Demmig-Adams, B., Adams, W.W., III: The role of xanthophyll cycle carotenoids in the protection of photosynthesis. — Trends Plant Sci. 1: 21–26, 1996.
Demmig, B., Björkmann, O.: Comparison of the effect of excessive light on chlorophyll fluorescence (77K) and photon yield of O2 evolution in leaves of higher plants. — Planta 171: 171–184, 1987.
Ebbs, S., Uchil, S.: Cadmium and zinc induced chlorosis in Indian mustard [Brassica juncea (L.) Czern] involves preferential loss of chlorophyll b. — Photosynthetica 46: 49–55, 2008.
Genty, B., Briantais J.-M., Baker, N.R.: The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. — Biochim. Biophys. Acta 990: 87–92, 1989.
Huner, N.P.A., Öquist, G., Hurry, V.M., Krol, M., Falk, S., Griffith, M.: Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. — Photosynth. Res. 37: 19–39, 1993.
Jin, E., Yokthongwattana, K., Polle, J.E.W., Melis, A.: Role of the reversible xanthophyll cycle in the photosystem II damage and repair cycle in Dunaliella salina. — Plant Physiol. 132: 352–364, 2003.
Johnston, J.A., Grise, D.J., Donovan, L.A., Arnold, M.L: Environment-dependent performance and fitness of Iris brevicaulis, I. fulva (Iridaceae), and hybrids. — Am. J. Bot. 88: 933–938, 2001.
Kovačik, J., Repčak, M., Kron, I.: Nitrogen deficiency induced changes of free amino acids and coumarin contents in the leaves of Matricaria chamomilla. — Acta Physiol. Plant. 28: 159–164, 2006.
Kouřil, R., Lazár, D. Lee, H., Jo, J., Nauš, J.: Moderately elevated temperature eliminates resistance of rice plants with enhanced expression of glutathione reductase to intensive photooxidative stress. — Photosynthetica 41: 571–578, 2003.
Kováčik, J., Klejdus, B., Hedbavny, J., Štork, F. Bačkor, M.: Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. — Plant Soil 320: 231–242, 2009.
Kráľová, K., Masarovičová, E., Ondrejkovičová, I., Bujdoš, M.: Effect of selenium oxidation state on cadmium translocation in chamomile plants. — Chem. Papers 61: 171–175. 2007.
Krause, G.H., Weis, E.: Chlorophyll fluorescence and photosynthesis. The basics. — Annu. Rev. Plant Physiol. 42: 313–349, 1991.
Kubát, K., Hrouda, L., Chrtek, J.,Jr., Kaplan, Z., Kirschner, Štěpánek, J. (ed.): [Key to the Flora of the Czech Republic]. — Akademia, Praha 2002. [In Czech.]
Lavaud, J., Kroth, P.G.: In diatoms, the transthylakoid proton gradient regulates the photoprotective non-photochemical fluorescence quenching beyond its control on the xanthophyll cycle. — Plant Cell Physiol. 47: 1010–1016, 2006.
Lichtenthaler, H.K., Wellburn, A.R.: Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. — Biochem. Soc. Trans. 11: 591–592, 1983.
Lichtenthaler, H.K., Ač, A., Marek, M.V., Kalina, J., Urban, O.: Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. — Plant Physiol. Biochem. 45: 577–588, 2006.
Lu, C.M., Lu, Q.T., Zhang, J.H., Zhang, Q., Kuang, T.Y.: Xantophyll cycle, light energy dissipation and photosystem II down-regulation in senescent leaves of wheat plants grown in the field. — Aust. J. Plant Physiol. 28: 1023–1030, 2001.
Montanaro, G., Dichio, B., Xiloyannis, C.: Response of photosynthetic machinery of field-grown kiwifruit under Mediterranean condition during drought and re-watering. — Photosynthetica 45: 533–540, 2007.
Maxwell, K., Johnson, G.N.: Chlorophyll fluorescence — a practical guide. — J. Exp. Bot. 51: 659–668, 2000.
Ohtsuka, T., Ito, H., Tanaka, A.: Conversion of chlorophyll b to chlorophyll a and the assembly of chlorophyll with apoproteins by isolated chloroplasts. — Plant Physiol. 113: 137–147. 1997.
Öquist, G., Hurry, V.M., Huner, N.P.A.: The temperature dependence of the redox state of QA and the susceptibility of photosynthesis to photoinhibition. — Plant Physiol. Biochem. 31: 683–691, 1993.
Ort, D.R: When there is too much light. — Plant Physiol. 125: 29–32, 2001.
Papadakis, I.E., Giannakoula A., Antonopoulou, C.P., Moustakas, M., Avramaki, E., Therios, I.N. Photosystem 2 activity of Citrus volkameriana (L.) leaves as affected by Mn nutrition and irradiance. — Photosynthetica 45: 208–213, 2007.
Pavlovič, A., Masarovičová, E., Kráľová, K., Kubová, J.: Response of chamomile plants (Matricaria recutita L.) to cadmium treatment. — Bull. Environ. Contamin. Toxic. 77: 763–771, 2006.
Proctor, M.C.F., Tuba, Z.: Poikilohydry and homoihydry: antithesis or spectrum of possibilities? — New Phytol. 156: 327–349, 2002.
Ramírez, D.A., Valladares, F., Domingo, F., Bellot, J.: Seasonal water-use efficiency and chlorophyll fluorescence response in alpha grass (Stipa tenacissima L.) is affected by tussock size. — Photosynthetica 46: 222–231, 2008.
Razmjoo, K., Heydarizadeh, P. Sabzalian, M.R.: Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomila. — Int. J. Agr. Biol. 10: 451–454, 2008.
Rutkowski, L. [Key to the Recognition of Vascular Plants of Lowland Poland.] PWN, Warsaw 1998. [In Pol.]
Šalamon, I.: The Slovak gene pool of German chamomile (Matricaria recutita L.) and comparison in its parameters. — HortSci. 2: 70–75, 2004.
Šalamon, I., Kráľová, K. Masarovičová, E.: Accumulation of cadmium in chamomile plants cultivated in Eastern Slovakia regions. — Acta Hort. 749: 217–222. 2007.
Schreiber, U., Bilger, W.: Progress in chlorophyll fluorescence research: major developments during the past years in retrospect. — Progr. Bot. 54: 151–173, 1993.
Schreiber, U., Schliwa, U., Bilger, W.: Continuous recording of photochemical and non-photochemical chlorophyll fluorescence with a new type of modulation fluorometer. — Photosynth Res. 10: 51–62, 1986.
Seidler-Łożykowska, K.: Chamomile cultivars and their cultivation in Poland. — Acta Hortic. 749: 111–114. 2007.
Seidler-Łożykowska, K.: [Comparison of some traits of chamomile strains and varieties with high content of alfabisabolol. Part II.] — Herba Pol. 1: 5–11, 2000. [In Pol.]
Sofo A., Dichio B., Montanaro G., Xiloyannis C.: Photosynthetic performance and light response of two olive cultivars under different water and light regimes. — Photosynthetica 47: 602–608, 2009.
Verhoeven, A.S., Demmig-Adams, B., Adams, W.W.: Enhanced employment of the xanthophylls cycle and thermal energy dissipation in spinach exposed to high light and N stress. — Plant Physiol. 113: 817–824, 1997.
Wentworth, M., Murchle, E.H., Gray, J.E., Villegas, D., Pastenes, C., Pinto, M., Horton, P.: Differential adaptation of two varieties of common bean to abiotic stress. II. Acclimation of photosynthesis. — J. Exp. Bot. 57: 699–709, 2006.
Xu, D.H., Li J.H., Fang, X.W., Wang, G., Su, P.X.: Photosynthetic activity of poikilochlorophyllous desiccation tolerant plant Reaumuria soongorica during dehydration and re-hydration. — Photosynthetica 46: 547–551, 2008.
Yamane, Y., Shikanai, T., Kashino, Y., Koike, H., Satoh, K.: Reduction of QA in the dark: another cause of fluorescence F0 increases by high temperatures in higher plants. — Photosynth. Res. 63: 23–34, 2000.
Zhang, Q., Chen, J.-W., Li, B.-G., Cao, K.-F.: The effect of drought on photosynthesis in two epiphytic and two terrestrial tropical fern species. — Photosynthetica 47: 128–132, 2009.
Acknowledgement
The authors thank to Ms Ewa Piechocka and Ms Ewa Przydanek (Institute of Natural Fibres and Medicinal Plants, Poznań) for excellent technical support. RBK will also to thank to the two anonymous reviewers for the comments which allowed to improve the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bączek-Kwinta, R., Kozieł, A. & Seidler-Łożykowska, K. Are the fluorescence parameters of German chamomile leaves the first indicators of the anthodia yield in drought conditions?. Photosynthetica 49, 87–97 (2011). https://doi.org/10.1007/s11099-011-0013-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-011-0013-3