Abstract
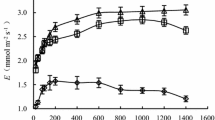
Gas exchange of Carex cinerascens was carried out in Swan Islet Wetland Reserve (29°48′ N, 112°33′ E). The diurnal photosynthetic course of C. cinerascens in the flooded and the nonflooded conditions were analyzed through the radial basis function (RBF) neural network approach to evaluate the influences of environmental variables on the photosynthetic activity. The inhibition of photosynthesis induced by soil flooding can be attributed to the reduced stomatal conductance (g s), the deficiency of Rubisco regeneration and decreased chlorophyll (Chl) content. As revealed by analysis of artificial neural network (ANN) models, g s was the dominant factor in determining the photosynthesis response. Weighting analysis showed that the effect of water pressure deficit (VPD) > air temperature (T) > CO2 concentration (C a) > air humidity (RH) > photosynthetical photon flux density (PPFD) for the nonflooded model, whereas for the flooded model, the factors were ranked in the order VPD > C a > RH > PPFD > T. The different photosynthetic response of C. cinerascens found between the nonflooded and flooded conditions would be useful to evaluate the flood tolerance at plant species level.
Similar content being viewed by others
Abbreviations
- ANN:
-
artificial neural network
- AQY:
-
apparent quantum yield
- C a :
-
CO2 concentration
- C i :
-
intercellular CO2 concentration
- CE:
-
carboxylation efficiency
- Chl:
-
chlorophyll
- E :
-
transpiration rate
- ETR:
-
electron transport rate
- Fm :
-
maximum fluorescence of dark state
- Fm′:
-
maximum fluorescence of light-adapted state
- Fo :
-
minimum fluorescence of dark state
- Fo′:
-
minimum fluorescence of light-adapted state
- Fs :
-
steady-state fluorescence
- Fv :
-
variable fluorescence
- Fv/Fm :
-
maximum quantum yield of PSII
- Fv/Fo :
-
the ratio of variable fluorescence to minimum fluorescence
- g s :
-
stomatal conductance
- Jmax :
-
the light saturated rate of electron transport
- Lc:
-
light compensation point
- Ls:
-
light saturation point
- P N :
-
net photosynthetic rate
- PAR:
-
photosynthetically active radiation
- PPFD:
-
photosynthetic photon flux density
- PSII:
-
photosystem II
- qN :
-
non-photochemical quenching coefficient
- qP :
-
photochemical quenching coefficient
- R D :
-
dark respiration rate
- R day :
-
day respiration
- RBF:
-
radial basis function
- RH:
-
air humidity
- Rubisco:
-
ribulose-1,5-bisphosphate carboxylase/oxygenase
- T:
-
air temperature
- Tl :
-
leaf temperature
- Vcmax :
-
maximum rate of carboxylation
- VPD:
-
water-pressure deficit
- WUE:
-
water-use efficiency
- ΦPSII :
-
effective quantum yield of PSII
- Γ:
-
CO2 compensation point
References
Balls, G.R., Palmer-Brown, D., Sanders, G.E.: Investigating microclimatic influences on ozone injury in clover (Trifolium subterraneum) using artificial neural networks. — New Phytol. 132: 271–286, 1996.
Baruch, Z.: Responses to drought and flooding in tropical forage grasses. II. Leaf water potential, photosynthesis rate and alcohol dehydrogenase activity. — Plant Soil 164: 97–105, 1994.
Bianchini, M., Frasconi, P., Gori, M.: Learning without local minima in radial basis function networks. — IEEE Trans. Neural Networks 6: 749–756, 1995.
Bradford, K.J.: Effects of soil flooding on leaf gas exchange of tomato plants. — Plant Physiol. 73: 475–479, 1983.
Bragina, T.V., Ponomareva, Y.V., Drozdova, I.S., Grinieva, G.M.: Photosynthesis and dark respiration in leaves of different ages of partly flooded maize seedlings. — Russ. J. Plant Physiol. 51: 342–347, 2004.
Brown, C.E., Pezeshki, S.R.: A study on waterlogging as a potential tool to control Ligustrum sinense populations in western Tennessee. — Wetlands 20: 429–437, 2000.
Chen, H.J., Qualls, R.G., Blank, R.R.: Effect of soil flooding on photosynthesis, carbohydrate partitioning and nutrient uptake in the invasive exotic Lepidium latifolium. — Aquat. Bot. 82: 250–268, 2005.
Farquhar, G.D., von Caemmerer, S.: Modeling of photosynthetic responses to environmental conditions. — In: Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H. (ed.): Physiological Plant Ecology. II.Water Relation and Carbon Assimilation. Pp. 549–587. Springer-Verlag, Berlin — Heidelberg — New York 1982.
Farquhar, G.D., von Caemmerer, S.V., Berry, J.A.: A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. — Planta 149: 78–90, 1980.
Genty, B., Briantais, J.M., Baker, N.R.: The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. — Biochim. Biophys. Acta 990: 87–92. 1989.
Gravatt, D.A., Kirby, C.J.: Patterns of photosynthesis and starch allocation in seedlings of four bottomland hardwood tree species subjected to flooding. — Tree Physiol. 18: 411–417, 1998.
Huntingford, C., Cox, P.M.: Use of statistical and neural network techniques to detect how stomatal conductance responds to changes in the local environment. — Ecol. Model. 97: 217–246, 1997.
Jeong, K.S., Joo, G.J., Kim, H.W., Ha, K., Recknagel, F.: Prediction and elucidation of phytoplankton dynamics in the Nakdong River (Korea) by means of a recurrent artificial neural network. — Ecol. Model. 146: 115–129, 2001.
Kozlowski, T.T.: Plant responses to flooding of soil. — Bioscience 34: 162–167, 1984.
Li, M., Yang, D., Li, W.: Leaf gas exchange characteristics and chlorophyll fluorescence of three wetland plants in response to long-term soil flooding — Photosynthetica 45: 222–228, 2007.
Liao, C.T., Lin, C.H.: Effect of flooding stress on photosynthetic activities of Momordica charantia. — Plant Physiol. Biochem. 32: 479–485, 1994.
Lichtenthaler, H.K.: Chlorophyll. and carotenoids, pigments of photosynthetic biomembranes. — Methods Enzymol. 148: 350–382, 1987.
Macek, P., Rejmánková, E., Houdková, K.: The effect of longterm submergence on functional properties of Eleocharis cellulosa Torr. — Aquat. Bot. 84: 251–258, 2006.
Marshall, B., Biscoe, P.V.: A model for C3 leaves describing the dependence of net photosynthesis on irradiance. Derivation. — J. Exp. Bot. 31: 29–39, 1980.
Mauchamp, A, Méthy, M.: Submergence-induced damage of photosynthetic apparatus in Phragmites australis. — Environ. Exp. Bot. 51: 227–235, 2004.
Maxwell, K., Johnson, G.N.: Chlorophyll fluorescence — a practical guide. — J. Exp. Bot. 51: 659–668, 2000.
McKevlin, M.R., Hook, D.D., McKee, W.H.: Growth and nutrient use efficiency of water tupelo seedlings in flooded and well drained soil. — Tree Physiol. 15: 753–758, 1995.
Melesse, A.M., Hanley, R.S.: Artificial neural network application for multi-ecosystem carbon flux simulation — Ecol. Model. 189: 305–314, 2005.
Mielke, M.S., de Almeida, A.-A.F., Gomes, F.P., Aguilar, M.A.G., Mangabeira, P.A.O.: Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. — Environ. Exp. Bot. 50: 221–231, 2003.
Mishra, S.K., Patro, L., Mohapatra, P.K., Biswal, B: Response of senescing rice leaves to flooding stress. — Photosynthetica 46: 315–317, 2008.
Mommer, L., Pons, T.L., Visser, E.J.W.: Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. — J. Exp. Bot. 57: 283–290, 2006.
Pallas, J.E., Kays, S.J.: Inhibition of photosynthesis by ethylene — a stomatal effect. — Plant Physiol. 70: 598–601, 1982.
Park, J., Sandberg, I.W.: Universal approximation using radial basis functions network. — Neural Computation 3: 246–257, 1991.
Pezeshki, S.R.: Responses of baldcypress (Taxodium distichum) seedlings to hypoxia: leaf protein content, ribulose-1, 5-bisphosphate carboxylase/oxygenase activity and photosynthesis. Photosynthetica 30: 59–68, 1994.
Pezeshki, S.R.: Wetland plant responses to soil flooding. — Environ. Exp. Bot. 46: 299–312, 2001.
Pezeshki, S.R, Chambers, J.L.: Responses of cherrybark oak seedlings to short-term flooding. — Forest Sci. 31: 760–771, 1985.
Pezeshki, S.R., Pardue, J.H., DeLaune, R.D.: The influence of soil oxygen deficiency on alcohol dehydrogenase activity, root porosity, ethylene production and photosynthesis in Spartina patens. — Environ. Exp. Bot. 33: 565–573, 1993.
Ponnamperuma, F.N.: Effects of flooding on soils. — In: Kozlowski, T.T.(ed.): Flooding and Plant Growth. Pp. 1–44. Academic Press, Orlando- San Diego — San Francisco — New York — London — Toronto — Montreal — Sydney — Tokyo — São Paulo 1984.
Poorter, H.: Do slow-growing species and nutrient-stressed plants respond relatively strongly to elevated CO2? — Glob. Change Biol. 4: 693–697, 1998.
Schreiber, U., Bilger, W., Neubauer, C.: Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. — In: Schulze, E.-D., Caldwell, M.M. (ed.): Ecophysiology of Photosynthesis. Pp. 49–70. Springer- Verlag, Berlin 1994.
Taylor, G.E., Gunderson, C.A.: Physiological site of ethylene effects on carbon dioxide assimilation in Glycine max. L. Merr. — Plant Physiol. 86: 85–92, 1988.
Wasserman, P.D.: Neural Computing: Theory and Practice, Van Nostrand Reinhold, New York 1989.
Yamori, W., Noguchi, K., Terashima, I.: Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. — Plant Cell Environ. 28: 536–547, 2005.
Yordanova, R.Y., Alexieva, V.S., Popova, L.P.: Influence of root oxygen deficiency on photosynthesis and antioxidant status in barley plants. — Russ. J. Plant Physiol. 50: 163–167, 2003.
Acknowledgement
This work was financed by Innovation Key project of CAS (O754551B 03), Innovation Key project of CAS (KSCX2-YW-Z-1023-5), grant (30700083) from Natural Sciences Foundation of China and project (CN2357) funded by WWF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, M., Hou, G., Yang, D. et al. Photosynthetic traits of Carex cinerascens in flooded and nonflooded conditions. Photosynthetica 48, 370–376 (2010). https://doi.org/10.1007/s11099-010-0048-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-010-0048-x