Abstract
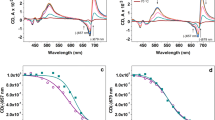
Typical chestnut thylakoid extracts isolated by mechanical disruption of leaf tissues had an equivalent of 0.28 kg m−3 chlorophyll (Chl) which is six times less than in thylakoids obtained from spinach, although Chl content in leaves was only half as small. According to optical microscopy, the vesicles showed a good integrity, exhibiting at 21 °C a high capacity of photon-induced potential membrane generation, which was demonstrated by the almost full 9-amino-6-chloro-2-methoxyacridine fluorescence quenching in a hyper-saline medium containing 150 mM KCl and having osmotic potential of −1.5 MPa. The half-time of the thylakoid potential generation was 11.7 s with the time of dissipation around 8.9 s. In such conditions, spinach thylakoids showed an increased swelling and also differences in the half-time generation which was almost four times faster than was observed in chestnut. However, when spinach thylakoids were incubated in a typical hypo-saline medium without KCl with osmotic potential −0.8 MPa, no additional swelling was observed. Consequently the half-time of potential dissipation was 35 s. Studies with nigericin suggested a chestnut thylakoid ΔpH significantly smaller than that observed in spinach, which was confirmed by the measurements of the ATP driven pumping activity.
Similar content being viewed by others
Abbreviations
- ACMA:
-
9-amino-6-chloro-2-methoxyacridine
- BHT:
-
butylated hydroxytoluene
- BSA:
-
bovine serum albumin
- EDTA:
-
disodium ethylenediamine tetraacetic acid
- F:
-
ACMA fluorescence quenching under dark conditions
- MV:
-
methyl viologen
- PS:
-
photosystem
- Q:
-
light-induced ACMA fluorescence quenching
- R:
-
ACMA fluorescence recuperation
- t0.5l:
-
half-time fluorescence quenching
- t0.5d:
-
half-time fluorescence recuperation
- Tricine:
-
N-tris (hydroxymethyl) methylglycine
- Δp:
-
electric membrane potential
- ΔpH:
-
electrochemical potential difference of protons
- Δψ:
-
transmembrane potential
- Ψπ :
-
osmotic potential
- Ψw :
-
water potential
References
Albert, A.: The Acridines — Their Preparation, Physical, Chemical and Biological Properties and Uses. — Edward Arnold, London 1966.
Bakker-Grunwald, T., van Dam, K.: On the mechanism of activation of the ATPase in chloroplasts. — Biochim. biophys. Acta 347: 290–298, 1974.
Barber, J.: Influence of surface charges on thylakoid structure and function. — Annu. Rev. Plant Physiol. 33: 261–295, 1982.
Barber, J.: Surface electrical charges and protein phosphorylation. — In: Staehelin, L.A., Arntzen, C.J. (ed.): Photosynthesis III. Pp. 653–664. Springer-Verlag, Berlin — Heidelberg — New York — Tokyo 1986.
Berkowitz, G.A.: Water and salt stress. — In: Raghavendra, A.S. (ed.): Photosynthesis. A Comprehensive Treatise. Pp. 226–237. Cambridge University Press, Cambridge 1998.
Brock, I.W., Mills, J.D., Robinson, D., Robinson, C.: The ΔpH-driven, ATP-independent protein translocation mechanism in the chloroplast thylakoid membrane. Kinetics and energetics. — J. biol. Chem. 270: 1657–1662. 1995.
Cortizo, E.V., Madrinan, M.L.V., Madrinan, F.J.V.: El Castano. [The Chestnut.] — Edilesa, Leon 1996. [In Portug.]
Crawford, M.: Chestnuts. Production and Culture. — Agroforestry Research Trust, Devon 1995.
Ewy, R.G., Dilley, R.A.: Distinguishing between luminal and localized proton buffering polls in thylakoid membranes. — Plant Physiol. 122: 583–595, 2000.
Fiolet, J.W.T., van der Erf ter Haar, L., Kraayenhof, R., van Dam, K.: On the stimulation of the light-induced proton uptake by uncoupling aminoacridine derivatives in spinach chloroplasts. — Biochim. biophys. Acta 387: 320–334, 1975.
Giersch, C., Heber, U., Kobayashi, Y., Inoue, Y., Shibata, K., Heldt, H.W.: Energy charge, phosphorylation potential and proton motive force in chloroplasts. — Biochim. biophys. Acta 590: 59–73, 1980.
Gilmore, A.M., Govindjee: How higher plants respond to excess light: Energy dissipation in photosystem II. — In: Singhal, G.S., Renger, G., Sopory, S.K., Irrgang, K.-D., Govindjee (ed.): Concepts in Photobiology: Pp. 513–548. Kluwer Academic Press, Boston — Dordrecht — London; Narosa Publ. House, Delhi — Madras — Bombay — Calcutta — London 1999.
Gomes-Laranjo, J., Peixoto, F., Sang, H., Kraayenhof, R., Torres-Pereira, J.: A comparative study on membrane potentials of chestnut (C. sativa Mill. cv Aveleira) and spinach thylakoids. — Biochim. biophys. Acta 1658(Suppl.): 254, 2004.
Gomes-Laranjo, J.C.E., Wong Fong Sang, H.W., Kraayenhof, R., Torres-Pereira, J.M.G.: Acclimation of chloroplasts from North-and South-exposed canopy sectors of chestnut (Castanea sativa Mill.). — Biochim. biophys. 1555(Suppl.): 236, 2002.
Hipkins, M.F., Baker, N.R.: Spectroscopy. — In: Hipkins, M.F., Baker, N.R. (ed.): Photosynthesis. Energy Transduction. A Practical Approach. Pp. 51–101. IRL Press, Oxford 1986.
Hurry, V., Huner, N., Selstam, E., Gardestrom, P., Oquist, G.: Photosynthesis at low growth temperatures. — In: Raghavendra, A.S. (ed.): Photosynthesis. A Comprehensive Treatise. Pp. 238–249. Cambridge University Press, Cambridge 1998.
Kraayenhof, R.: Analysis of membrane architecture: fluorimetric approach. — In: Colowick, S.P., Kaplan, N.O. (ed.): Methods in Enzymology. Vol. 69. Pp. 510–520. Academic Press, New York — London — Toronto — Sydney — San Francisco 1980.
Kraayenhof, R., Sterk, G.-J., Wong Fong Sang, H.W.: Probing biomembrane interfacial potential and pH profiles with a new type of float-like fluorophores positioned at varying distance from the membrane surface. — Biochemistry 32: 10057–10066, 1993.
Kraayenhof, R., Sterk, C.J., Wong Fong Sang, H.W., Krab, K., Epand, R.M.: Monovalent cations differentially affect membrane surface properties and membrane curvature, as revealed by fluorescent probes and dynamic light scattering. — Biochim. biophys. Acta 1282: 293–302, 1996.
Kraayenhof, R., Torres-Pereira, J.M.G., Peters, F.A.L.J., Wong Fong Sang, H.W.: Temperature dependence of electrical events in thylakoid membranes: comparison of membrane potential, surface potential and aminoacridine binding. — In: Sybesma, C. (ed.): Advances in Photosynthesis Research. Vol. II. Pp. 289–292. Martinus Nijhoff/Dr W. Junk Publ., The Hague — Boston — Lancaster 1984.
Leegood, R.C., Malkin, R.: Photophosphorylation. — In: Hipkins, M.F., Baker, N.R. (ed.): Photosynthesis. Energy Transduction. A Practical Approach. Pp. 9–26. IRL Press, Oxford 1986.
Lichtenthaler, H.K.: Chlorophylls and carotenoids — pigments of photosynthetic biomembranes. — In: Colowick, S.P., Kaplan, N.O. (ed.): Methods in Enzymology. Vol. 148. Pp. 350–382. Academic Press, San Diego — New York — Berkeley — Boston — London — Sydney — Tokyo — Toronto 1987.
Mills, J.D.: Photophosphorylation. — In: Hipkins, M.F., Baker, N.R. (ed.): Photosynthesis. Energy Transduction. A Practical Approach. Pp. 143–187. IRL Press, Oxford 1986.
Murakami, S., Packer, L.: Reversible changes in the conformation of thylakoid membranes accompanying chloroplast contraction or expansion. — Biochim. biophys. Acta 180: 420–423, 1969.
Nakatani, H.Y., Barber, J., Forrester, J.A.: Surface charges on chloroplast membranes as studied by particle electrophoresis. — Biochim. biophys. Acta 504: 215–225, 1978.
Packer, L., Torres-Pereira, J.M.G., Chang, P., Hansen, S.: Stabilization of chloroplast membranes as measured by light-induced quenching of acridine dyes. — In: Avron, M. (ed.): Proceedings of the Third International Congress on Photosynthesis. Vol. II. Pp. 867–872. Elsevier, Amsterdam — Oxford — New York 1975.
Rottenberg, H.: The measurement of membrane potential and ΔpH in cells, organelles, and vesicles. — In: Packer, L., Fleischer, S. (ed.): Biomembranes. Pp. 625–648. Academic Press, San Diego 1997.
Salisbury, F.B., Ross, C.W.: Plant Physiology. — Wadsworth Publishing Company, Belmont 1992.
Schuldiner, S., Rottenberg, H., Avron, M.: Determination of ΔpH in chloroplasts. 2. Fluorescence amines as a probe for the determination of ΔpH in chloroplasts. — Eur. J. Biochem. 25: 64–70, 1972.
Schuurmans, J.J., Casey, R.P., Kraayenhof, R.: Transmembrane electrical potential formation in spinach chloroplasts. Investigation using a rapidly-responding extrinsic probe. — FEBS Lett. 94: 405–409, 1978.
Schuurmans, J.J., Kraayenhof, R., Leeuwerik, L.J., Veen, J.P.C., Marun, D. van, Jasper, C.G.G.: A thermoelectrically regulated multipurpose cuvette for simultaneous time-dependent measurements. — Anal. Biochem. 127: 93–99, 1982.
Strotmann, H., Shavit, N.: Photophosphorylation. — In: Singhal, G.S., Renger, G., Sopory, S.K., Irrgang, K.-D., Govindjee (ed.): Concepts in Photobiology. Pp. 389–430. Kluwer Academic Publ., Boston — Dordrecht — London; Narosa Publ. House, Delhi — Madras — Bombay — Calcutta — London 1999.
Torres-Pereira, J.M.: Preservation of chloroplast membranes in vitro. — In: Burton, R.M., Packer, L. (ed.): Biomembranes-Lipids, Proteins and Receptors. Pp. 1–9. BI-Science Publications Division, Webster Groves 1974.
Torres-Pereira, J., Mehlhorn, R., Keith, A.D., Packer, L.: Changes in membrane lipid structure of illuminated chloroplasts-Studies with spin-labeled and freeze-fractured membranes. — Arch. Biochem. Biophys. 160: 90–99, 1974a.
Torres-Pereira, J.M., Takaoki, T., Packer, L.: Factors affecting the stability of chloroplast membrane in vitro. — Biochim. biophys. Acta 352: 260–267, 1974b.
Torres-Pereira, J.M.G, Wong Fong Sang, H., Theuvenet, A.P.R., Kraayenhof, R.: Electric surface charge dynamics of chloroplasts thylakoid membranes. Temperature dependence of electrokinetic potential and aminoacridine interaction. — Biochim. biophys. Acta 767: 295–303, 1984.
Trissl, H.-W., Wilhelm, C.: Why do thylakoid membranes from higher plants form grana stacks? — Trends biochem. Sci. 18: 415–419, 1993.
Wang, A.Y.-L., Packer, L.: Mobility of membrane particles in chloroplasts. — Biochim. biophys. Acta 305: 488–492, 1973.
Walker, D.A., Cerovic, Z.G., Robinson, S.P.: Isolation of intact chloroplasts: general principles and criteria of integrity. — In: Packer, L., Fleischer, S. (ed.): Biomembranes. Pp. 173–186. Academic Press, San Diego 1997.
Witt, H.T.: Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The control role of the electric field. — Biochim. biophys. Acta 505: 355–427, 1979.
Witt, H.T.: Functional mechanism of water splitting photosynthesis. — Photosynth. Res. 29: 55–77, 1991.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomes-Laranjo, J., Salgado, P., Wong Fong Sang, H.W. et al. Isolation of chestnut chloroplasts: membrane potentials of chestnut and spinach thylakoids. Photosynthetica 43, 237–246 (2005). https://doi.org/10.1007/s11099-005-0039-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11099-005-0039-5