Abstract
Background
Although nivolumab has shown clinical benefits for relapsed malignant mesothelioma, its cost-effectiveness requires further investigation.
Aim
This study aimed to evaluate the cost-effectiveness of nivolumab compared to placebo for relapsed malignant mesotheliomas from the perspective of the Chinese healthcare system.
Method
A three-state Markov model was developed based on data from the phase 3 randomized CONFIRM clinical trial. The drug cost and utility values for the health state were obtained from the relevant literature. The measured outcomes included quality-adjusted life-years (QALYs) and incremental cost-effectiveness ratio (ICER). Probabilistic and one-way sensitivity analyses (OWSA) were performed to assess the uncertainty of the model.
Results
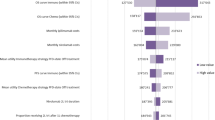
Patients receiving nivolumab gained more health benefits (0.65 QALYs vs. 0.43 QALYs). The cost was higher ($25,806.08 vs. $9,310.74) than for patients in the placebo group, resulting in an ICER of $75,805.11/QALY, which was above the willingness-to-pay (WTP) threshold of three times per capita GDP ($35,864.61) in China. The result of OWSA indicated that the cost of nivolumab, the utility of the disease progression, and the discount rate were the most significant factors. Probabilistic sensitivity analysis suggested that the probability that nivolumab was not cost-effective as was 100.00% above the specified WTP threshold.
Conclusion
From the perspective of the Chinese healthcare system, nivolumab was not as cost-effective as placebo for relapsed malignant mesothelioma.




Similar content being viewed by others
References
Pan D, Wang M, Liu W, et al. Clinical-pathological characteristics and prognostic factors for malignant peritoneal mesothelioma in the elderly. BMC Gastroenterol. 2022;22(1):292.
Scherpereel A, Opitz I, Berghmans T, et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J. 2020;55(6):1.
Shavelle R, Vavra-Musser K, Lee J, et al. Life expectancy in pleural and peritoneal mesothelioma. Lung Cancer Int. 2017;2017:2782590.
Peters S, Scherpereel A, Cornelissen R, et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann Oncol. 2022;33(5):488–99.
Sahu RK, Ruhi S, Jeppu AK, et al. Malignant mesothelioma tumours: molecular pathogenesis, diagnosis, and therapies accompanying clinical studies. Front Oncol. 2023;13:1204722.
Mansfield AS, Roden AC, Peikert T, et al. B7–H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014;9(7):1036–40.
Chapel DB, Stewart R, Furtado LV, et al. Tumor PD-L1 expression in malignant pleural and peritoneal mesothelioma by Dako PD-L1 22C3 pharmDx and Dako PD-L1 28–8 pharmDx assays. Hum Pathol. 2019;87:11–7.
Okada M, Kijima T, Aoe K, et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, Japanese phase II study in malignant pleural mesothelioma (MERIT). Clin Cancer Res. 2019;25(18):5485–92.
Quispel-Janssen J, van der Noort V, de Vries JF, et al. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol. 2018;13(10):1569–76.
Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol. 2019;20(2):239–53.
Fennell DA, Ewings S, Ottensmeier C, et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021;22(11):1530–40.
Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25(1):3–9.
Murray CJ, Evans DB, Acharya A, et al. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9(3):235–51.
Guyot P, Ades AE, Ouwens M, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:13.
Li W, Guo H, Li L, et al. Comprehensive comparison between adjuvant targeted therapy and chemotherapy for EGFR-mutant NSCLC patients: a cost-effectiveness analysis. Front Oncol. 2021;11: 619376.
Lin YT, Chen Y, Liu TX, et al. Cost-effectiveness analysis of camrelizumab immunotherapy versus docetaxel or irinotecan chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma. Cancer Manag Res. 2021;13:8219–30.
Wu B, Li T, Cai J, et al. Cost-effectiveness analysis of adjuvant chemotherapies in patients presenting with gastric cancer after D2 gastrectomy. BMC Cancer. 2014;14:984.
Shu Y, Tang Y, Ding Y, et al. Cost-effectiveness of nivolumab versus sorafenib as first-line treatment for advanced hepatocellular carcinoma. Int Immunopharmacol. 2023;122: 110543.
Wen F, Zheng H, Zhang P, et al. Atezolizumab and bevacizumab combination compared with sorafenib as the first-line systemic treatment for patients with unresectable hepatocellular carcinoma: a cost-effectiveness analysis in China and the United states. Liver Int. 2021;41(5):1097–104.
Li Y, Liang X, Li H, et al. Nivolumab versus sorafenib as first-line therapy for advanced hepatocellular carcinoma: a cost-effectiveness analysis. Front Pharmacol. 2022;13: 906956.
Scherpereel A, Antonia S, Bautista Y, et al. First-line nivolumab plus ipilimumab versus chemotherapy for the treatment of unresectable malignant pleural mesothelioma: patient-reported outcomes in CheckMate 743. Lung Cancer. 2022;167:8–16.
Zhan M, Zheng H, Xu T, et al. Cost-effectiveness analysis of additional bevacizumab to pemetrexed plus cisplatin for malignant pleural mesothelioma based on the MAPS trial. Lung Cancer. 2017;110:1–6.
Yang L, Cao X, Li N, et al. Cost-effectiveness analysis of nivolumab plus ipilimumab versus chemotherapy as the first-line treatment for unresectable malignant pleural mesothelioma. Ther Adv Med Oncol. 2022;14:17588359221116604.
Ye ZM, Tang ZQ, Xu Z, et al. Cost-effectiveness of nivolumab plus ipilimumab as first-line treatment for American patients with unresectable malignant pleural mesothelioma. Front Public Health. 2022;10: 947375.
Xu K, Wu H, Zhou C, et al. Cost-effectiveness of toripalimab plus chemotherapy for advanced esophageal squamous cell carcinoma. Int J Clin Pharm. 2023;45(3):641–9.
Zhu Y, Liu K, Qin Q, et al. Serplulimab plus chemotherapy as first-line treatment for extensive-stage small-cell lung cancer: A cost-effectiveness analysis. Front Immunol. 2022;13:1044678.
Acknowledgements
None.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lang, W., Wei, J., Jiang, Q. et al. Cost-effectiveness analysis of nivolumab versus placebo for relapsed malignant mesothelioma. Int J Clin Pharm 46, 158–165 (2024). https://doi.org/10.1007/s11096-023-01662-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01662-1