Abstract
Background
The results of the KHBO1401-MITSUBA trial suggest the effectiveness of triple therapy using gemcitabine, cisplatin, and S-1; however, the cost-effectiveness of this treatment regimen remains unclear.
Aim
We conducted a cost-utility analysis comparing triple therapy using gemcitabine, cisplatin, and S-1 with doublet therapy using gemcitabine and cisplatin for advanced biliary tract cancer from the perspective of a Japanese healthcare payer to investigate the economic sustainability of healthcare interventions.
Method
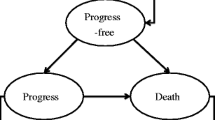
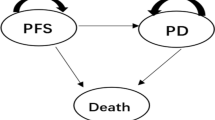
Based on the results of the KHBO1401-MITSUBA clinical trial, a partitioned survival model set over a 10-year time horizon was developed. Cost and utility data were sourced from previous studies. Health outcomes were measured as quality-adjusted life years (QALYs). Direct medical costs included drug costs and medical fees. The uncertainty and robustness of the model were evaluated using one-way and probabilistic sensitivity analyses. The willingness-to-pay threshold was set at 7.5 million Japanese yen (68,306 US dollars).
Results
Base case analysis revealed an incremental cost-effectiveness ratio for triple therapy at 4,458,733 Japanese yen (40,608 US dollars) per QALY. One-way sensitivity analysis showed that the parameter variation in the overall survival curves for each therapy had impacts exceeding the threshold. According to probabilistic sensitivity analysis, triple therapy had an 83.1% chance of being cost-effective at the threshold, and the 95% credible interval for the incremental cost-effectiveness ratio was 4,382,972-4,514,257 JPY (39,918–41,113 US dollars).
Conclusion
Triple therapy using gemcitabine, cisplatin, and S-1 is cost-effective for the primary treatment of biliary tract cancer in the Japanese healthcare system.



Similar content being viewed by others
References
Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl5):v28-37. https://doi.org/10.1093/annonc/mdw324.
Rizzo A, Brandi G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15:547–54. https://doi.org/10.1080/17474124.2021.1890031.
Bridgewater JA, Goodman KA, Kalyan A, et al. Biliary tract cancer: Epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ Book. 2016;35:e194–203. https://doi.org/10.1200/EDBK_160831.
Cancer statistics. Japan: Cancer Information Service, National Cancer Center (Vital Statistics of Japan, Ministry of Health, Labour and Welfare). https://ganjoho.jp/reg_stat/statistics/data/dl/index.html#a7. Accessed 16 Oct 2022.
Ishihara S, Horiguchi A, Miyakawa S, et al. Biliary tract cancer registry in Japan from 2008 to 2013. J Hepatobiliary Pancreat Sci. 2016;23:149–57. https://doi.org/10.1002/jhbp.314.
Elvevi A, Laffusa A, Scaravaglio M, et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann Hepatol. 2022;27:100737. https://doi.org/10.1016/j.aohep.2022.100737
Mirallas O, López-Valbuena D, García-Illescas D, et al. Advances in the systemic treatment of therapeutic approaches in biliary tract cancer. ESMO Open. 2022;7:100503.
Primrose JN, Neoptolemos J, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–73. https://doi.org/10.1016/S1470-2045(18)30915-X.
Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for Biliary Tract Cancer. N Engl J Med. 2010;362:1273–81. https://doi.org/10.1056/NEJMoa0908721.
Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–74. https://doi.org/10.1038/sj.bjc.6605779.
Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30:1950–8. https://doi.org/10.1093/annonc/mdz402.
Ioka T, Kanai M, Kobayashi S, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 versus gemcitabine, cisplatin for advanced biliary tract cancer (KHBO1401- MITSUBA). J Hepatobiliary Pancreat Sci. 2023;30:102–10. https://doi.org/10.1002/jhbp.1219.
Nagino M, Hirano S, Yoshitomi H et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd. English ed. J Hepato Biliary Pancreatic Sci. ISBN: 0000000329118; 2021
Center for Outcomes Research and economic evaluation for health. Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical Council version 3.0.; 2022. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. Accessed 16 Oct 2022
O.E.C.D. Data. Exchange rates. https://data.oecd.org/conversion/exchange-rates.htm#indicator-chart. Accessed 16 Oct 2022
Japanese Society of Nephrology. Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012 [in Japanese]. Nihon Jinzo Gakkai Shi. 2012;54:1034–191.
Reimbursement schedule of social insurance. 2022. Tokyo, Japan: Ministry of Health, Labor and Welfare. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000188411_00037.html. Accessed 16 Oct 2022.
National health insurance drug price. Standard. Tokyo, Japan: Jiho Inc. ISBN: 9784840754040; 2022.
Hayashida K, Murakami G, Matsuda S, et al. History and profile of diagnosis Procedure Combination (DPC): development of a real data collection system for acute inpatient care in Japan. J Epidemiol. 2021;31:1–11. https://doi.org/10.2188/jea.JE20200288.
Ministry of Health., Labour and Welfare, Diagnostic Procedure Classification (DPC) electronic score table. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000198757_00004.html. Accessed 16, Oct 2022.
Miyake O, Murata K, Tanaka S, et al. Costs associated with febrile neutropenia in japanese patients with primary breast cancer: post-hoc analysis of a randomized clinical trial. Jpn J Clin Oncol. 2018;48:410–6. https://doi.org/10.1093/jjco/hyy030.
Kato K, Fukuda H. Comparative economic evaluation of home-based and hospital-based palliative care for terminal cancer patients. Geriatr Gerontol Int. 2017;17:2247–54. https://doi.org/10.1111/ggi.12977.
Kanai M, Hatano E, Kobayashi S, et al. A multi-institution phase II study of gemcitabine/cisplatin/S-1 (GCS) combination chemotherapy for patients with advanced biliary tract cancer (KHBO 1002). Cancer Chemother Pharmacol. 2015;75:293–300. https://doi.org/10.1007/s00280-014-2648-9.
Tikhonova IA, Huxley N, Snowsill T, et al. Economic analysis of first-line treatment with cetuximab or panitumumab for RAS wild-type metastatic colorectal cancer in England. Pharmacoeconomics. 2018;36(7):837–51. https://doi.org/10.1007/s40273-018-0630-9.
Guyot P, Ades AE, Ouwens MJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. https://doi.org/10.1186/1471-2288-12-9.
Sgouros J, Aravantinos G, Koliou GA, et al. First line gemcitabine/pazopanib in locally advanced and/or metastatic biliary tract carcinoma. A hellenic cooperative oncology group phase II study. Anticancer Res. 2020;40:929–38. https://doi.org/10.21873/anticanres.14026.
Chen R, Zhang Y, Lin K, et al. Cost-effectiveness analysis of capecitabine plus oxaliplatin versus gemcitabine plus oxaliplatin as first-line therapy for advanced biliary tract cancers. Front Pharmacol. 2022;13:871262. https://doi.org/10.3389/fphar.2022.871262.
Roth JA, Carlson JJ. Cost-effectiveness of gemcitabine + cisplatin vs. gemcitabine monotherapy in advanced biliary tract cancer. J Gastrointest Cancer. 2012;43:215–23. https://doi.org/10.1007/s12029-010-9242-0.
Tsukiyama I, Ejiri M, Yamamoto Y, et al. A cost-effectiveness analysis of gemcitabine plus cisplatin versus gemcitabine alone for treatment of advanced biliary tract cancer in Japan. J Gastrointest Cancer. 2017;48:326–32. https://doi.org/10.1007/s12029-016-9885-6.
Funding
No specific funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kashiwa, M., Matsushita, R. Model-based cost-utility analysis of gemcitabine, cisplatin, and S-1 as triple therapy for advanced biliary tract cancer. Int J Clin Pharm 45, 875–883 (2023). https://doi.org/10.1007/s11096-023-01580-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01580-2