Abstract
Background
Emicizumab is the latest treatment for patients with hemophilia A. Its safety in real-world data is limited, and regulatory agencies and clinical researchers have raised concerns about the risk of adverse events.
Aim
This study aimed to detect potential adverse event signals of emicizumab using the FDA Adverse Event Reporting System (FAERS) database.
Method
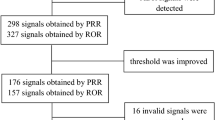
Data in FAERS from the fourth quarter of 2017 to the second quarter of 2021 were searched. Cases of adverse events were extracted using the Preferred Term in the Medical Dictionary for Regulatory Activities (version 24.0). Disproportionality analysis was performed using the reporting odds ratio (ROR) and information component (IC) methods based on statistical shrinkage transformation.
Results
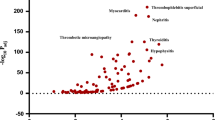
A total of 5,598,717 patients were included, of which 1,244 took emicizumab. A total of 703 emicizumab-related adverse event signals were mined, and 101 positive signals were detected. Haemarthrosis (ROR/ROR975/ROR025 = 155.62/184.34/131.38, IC/IC975/IC025 = 7.28/7.48/7.01), haemorrhage (ROR/ROR975/ROR025 = 71.01/81.18/62.12, IC/IC975/IC025 = 6.15/6.31/5.94), muscle haemorrhage (ROR/ROR975/ROR025 = 53.38/75.83/37.58, IC/IC975/IC025 = 5.74/6.16/5.15), traumatic haemorrhage (ROR/ROR975/ROR025 = 27.78/46.29/16.67, IC/IC975/IC025 = 4.80/5.40/3.92), haematoma (ROR/ROR975/ROR025 = 18.15/26.35/12.51, IC/IC975/IC025 = 4.18/4.63/3.55), device-related thrombosis (ROR/ROR975/ROR025 = 21.27/37.57/12.04, IC/IC975/IC025 = 4.41/5.08/3.43), and activated partial thromboplastin time prolonged (ROR/ROR975/ROR025 = 20.68/36.51/11.71, IC/IC975/IC025 = 4.37/5.04/3.39) had the strongest signal intensities. Haemorrhage, haemarthrosis, arthralgia, fall, and injection site pain were reported more frequently.
Conclusion
This study found that mild arthralgia and injection site reaction were associated with emicizumab. Attention should also be paid to other serious adverse events related to emicizumab, such as acute myocardial infarction and sepsis, to ensure patient safety.
Similar content being viewed by others
References
Thrombosis and Hemostasis Group, Hematology Society of Chinese Medical Association; Hemophilia Treatment Center Collaborative Network of China. [Consensus of Chinese expert on the diagnosis and treatment of hemophilia (version 2017)]. Zhonghua Xue Ye Xue Za Zhi. 2017 May 14;38(5):364 – 70.
Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Treatment Guidelines Working Group on Behalf of The World Federation Of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47.
Fischer K, Lewandowski D, Marijke van den Berg H, et al. Validity of assessing inhibitor development in haemophilia PUPs using registry data: the EUHASS project. Haemophilia. 2012;18(3):e241-6.
Yoneyama K, Schmitt C, Kotani N, et al. Pharmacometric approach to substitute for a conventional dose-finding study in rare diseases: example of phase iii dose selection for emicizumab in hemophilia A. Clin Pharmacokinet. 2018;57(9):1123–34.
Lenting PJ, Denis CV, Christophe OD. Emicizumab, a bispecific antibody recognizing coagulation factors IX and X: how does it actually compare to factor VIII? Blood. 2017;130(23):2463–8.
Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377(9):809–18.
Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs. 2018;10(2):183–203.
FDA approves emicizumab-kxwh for prevention and reduction of bleeding in patients with hemophilia A with factor VIII inhibitors. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-emicizumab-kxwh-prevention-and-reduction-bleeding-patients-hemophilia-factor-viii#:~:text=On%20November%2016%2C%202017%2C%20the,deficiency)%20with%20factor%20VIII%20inhibitors. Accessed 02 Oct 2022.
FDA Grants Roche Breakthrough Therapy Designation on Hemophilia Drug. https://www.biopharminternational.com/view/fda-grants-roche-breakthrough-therapy-designation-hemophilia-drug. Accessed 02 Oct 2022.
Rodriguez EM, Staffa JA, Graham DJ. The role of databases in drug postmarketing surveillance. Pharmacoepidemiol Drug Saf. 2001;10(5):407–10.
Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med. 2005;165(12):1363–9.
Questions. and Answers on FDA’s Adverse Event Reporting System (FAERS). https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. Accessed 02 Oct 2022.
van Puijenbroek EP, Bate A, Leufkens HG, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10.
Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315–21.
Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22(1):57–69.
Wang J, Ye XF, Guo XJ, et al. Exploration of statistical shrinkage parameters of disproportionality methods in spontaneous reporting system of China. Pharmacoepidemiol Drug Saf. 2015;24(9):962–70.
Almenoff JS, Pattishall EN, Gibbs TG, et al. Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther. 2007;82(2):157–66.
Kubota K, Koide D, Hirai T. Comparison of data mining methodologies using Japanese spontaneous reports. Pharmacoepidemiol Drug Saf. 2004;13(6):387–94.
Mannucci PM, Tuddenham EG. The hemophilias—from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773–9.
Mahlangu J, Oldenburg J, Paz-Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379(9):811–22.
Young G, Liesner R, Chang T, et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134(24):2127–38.
Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open-label, non-randomised phase 3 study. Lancet Haematol. 2019;6(6):e295–305.
Rodriguez-Merchan EC, Valentino LA. Emicizumab. Review of the literature and critical appraisal. Haemophilia. 2019;25(1):11–20.
Dane KE, Lindsley JP, Streiff MB, et al. Successful use of emicizumab in a patient with refractory acquired hemophilia A and acute coronary syndrome requiring percutaneous coronary intervention. Res Pract Thromb Haemostasis. 2019;3(3):420–3.
Chen ZP, Li G, Li ZK, et al. Effect of emicizumab on coagulation assays of hemophilia A. J Clin Hematol. 2020;33(11):771–5.
Bowyer A, Kitchen S, Maclean R. Effects of emicizumab on APTT, one-stage and chromogenic assays of factor VIII in artificially spiked plasma and in samples from haemophilia A patients with inhibitors. Haemophilia. 2020;26(3):536–42.
Knoebl P, Thaler J, Jilma P, et al. Emicizumab for the treatment of acquired hemophilia A. Blood. 2021;137(3):410–9.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761083s000lbl.pdf. Accessed 02 Oct 2022.
Li JY, Zhang YK, Zhang SY, et al. A new drug for hemophilia A: emicizumab. Practical Pharm Clin Remedies. 2019;22(10):1112–6.
Hooimeijer HL, Lukens MV, Verhagen MV, et al. A boy with joint pain associated with emicizumab treatment: the importance of plasma level measurement. Haemophilia. 2020;26(3):e138–40.
Acknowledgements
We thank for Dr Yuchen Qin for his help in the language polish of the manuscript revision.
Funding
This study was supported by the China Natural Science Foundation (No. 82073671), the Leading Talents of Public Health in Shanghai (No. GWV-10.2-XD22), the Excellent Young Scholars of Public Health in Shanghai (No. GWV-10.2-YQ33), the Construction Plan of Key Disciplines of Public Health system in Shanghai (GWV-10.1-XK05), and the Military Key Discipline Construction Project (Health Service-Naval Health Service Organization and Command) (No.03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Jinfang Xu and Xiaofei Ye have contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, L., Tian, Y., Chen, X. et al. Data mining and analysis for emicizumab adverse event signals based on the Food and Drug Administration Adverse Event Reporting System database. Int J Clin Pharm 45, 622–629 (2023). https://doi.org/10.1007/s11096-022-01514-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01514-4