Abstract
Background
Vancomycin area under the curve/minimum inhibitory concentration (AUC/MIC) has been proposed as a therapeutic drug monitoring (TDM) target to dose vancomycin. It is time-consuming to estimate AUCs using traditional methods. A two-point trough-peak method is more straightforward for calculating the vancomycin AUC. However, the technique and the AUC/MIC target have not been validated in Chinese patients.
Aim
To compare the clinical outcomes of vancomycin therapy in Chinese older adults (aged > 60 years) between the trough-only and the two-point peak-trough AUC TDM approaches.
Method
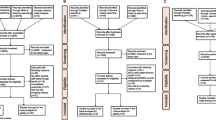
The patients were divided into study and control groups according to TDM approaches. A trough-based TDM was used in the control group (target trough level 15–20 mg/L). Stanford University has provided a method to predict vancomycin AUC using peak-valley concentration alone (two-point method). A two-point trough-peak TDM approach was employed in the study group (target AUC/MIC ≥ 400). The effect of vancomycin was evaluated in terms of clinical findings, laboratory values, and bacteriologic responses. The effects of treatment and kidney functions were compared between the two groups.
Results
A total of 389 patients met the study inclusion criteria, and 189 were excluded based on the exclusion criteria. Of the 200 patients, 80 received the two-point TDM approach (the study group), and 120 were monitored using the trough-based approach (the control group). The average age was 69.8 ± 7.1 years. Staphylococcus aureus (34%) was the most common Gram-positive bacteria. No vancomycin-related nephrotoxicity was observed in either group. The percentages of patients with an excellent response to vancomycin therapy were significantly higher in the study group than in the control group, 90% (72/80) versus 73.3% (88/120), P = 0.0039.
Conclusion
The two-point peak-trough method is practical for obtaining vancomycin AUC. The AUC/MIC ≥ 400 target demonstrates treatment effectiveness and safety in older Chinese patients.
Similar content being viewed by others
References
Stogios PJ, Savchenko A. Molecular mechanisms of vancomycin resistance. Protein Sci. 2020;29(3):654–69.
Oda K, Jono H, Nosaka K, et al. Reduced nephrotoxicity with vancomycin therapeutic drug monitoring guided by area under the concentration-time curve against a trough 15–20 μg/mL concentration. Int J Antimicrob Agents. 2020;56(4): 106109.
Drennan PG, Begg EJ, Gardiner SJ, et al. The dosing and monitoring of vancomycin: what is the best way forward? Int J Antimicrob Agents. 2019;53(4):401–7.
Kabbara WK, El-Khoury G, Chamas NR. Prospective evaluation of vancomycin therapeutic usage and trough levels monitoring. J Infect Dev Ctries. 2018;12(11):978–84.
Aljefri DM, Avedissian SN, Rhodes NJ, et al. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881–7.
Suchánková H, Lečbychová K, Strojil J, et al. Individualized dosing of vancomycin in geriatric patients. Epidemiol Mikrobiol Imunol. 2020;69(4):172–80.
Barber KE, Bell AM, Stover KR, et al. Intravenous vancomycin dosing in the elderly: a focus on clinical issues and practical application. Drugs Aging. 2016;33(12):845–54.
Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-e1317.
Lee BV, Fong G, Bolaris M, et al. Cost-benefit analysis comparing trough, two-level AUC and Bayesian AUC dosing for vancomycin. Clin Microbiol Infect. 2021;27(9):1346.e1-1346.e7.
Meng L, Wong T, Huang S, et al. Conversion from vancomycin trough concentration-guided dosing to area under the curve-guided dosing using two sample measurements in adults: implementation at an Academic Medical Center. Pharmacotherapy. 2019;39(4):433–42.
Álvarez R, López Cortés LE, Molina J, et al. Optimizing the clinical use of vancomycin. Antimicrob Agents Chemother. 2016;60(5):2601–9.
Drennan PG, Begg EJ, Gardiner SJ, et al. The dosing and monitoring of vancomycin: what is the best way forward? Int J Antimicrob Agents. 2019;53(4):401–7.
Al-Sulaiti FK, Nader AM, Saad MO, et al. Clinical and pharmacokinetic outcomes of peak-trough-based versus trough-based vancomycin therapeutic drug monitoring approaches: a pragmatic randomized controlled trial. Eur J Drug Metab Pharmacokinet. 2019;44(5):639–52.
Shah DN, Bhatt NS, Welch JK, et al. Defining acute renal dysfunction as a criterion for the severity of Clostridium difficile infection in patients with community-onset vs hospital-onset infection. J Hosp Infect. 2013;83(4):294–9.
Ahmed MO, Baptiste KE. Vancomycin-resistant enterococci: a review of antimicrobial resistance mechanisms and perspectives of human and animal health. Microb Drug Resist. 2018;24(5):590–606.
Lodise TP, Drusano G. Vancomycin area under the curve-guided dosing and monitoring for adult and pediatric patients with suspected or documented serious methicillin-resistant Staphylococcus Aureus infections: putting the safety of our patients first. Clin Infect Dis. 2021;72(9):1497–501.
Cusumano JA, Klinker KP, Huttner A, et al. Towards precision medicine: Therapeutic drug monitoring-guided dosing of vancomycin and β-lactam antibiotics to maximize effectiveness and minimize toxicity. Am J Health Syst Pharm. 2020;77(14):1104–12.
Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82–98.
Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of Vancomycin for serious methicillin-resistant Staphylococcus Aureus infections: a revised consensus guideline and review by the American society of health-system pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2020;71(6):1361–4.
Acknowledgements
None.
Funding
This study was supported by the Natural Science Foundation of Fujian Province, China (Grant No. 2020J011094).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Lin, X., Wang, L. et al. Clinical validation of the two-point method for predicting vancomycin AUC based on peak and trough plasma concentrations. Int J Clin Pharm 44, 1325–1331 (2022). https://doi.org/10.1007/s11096-022-01474-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01474-9