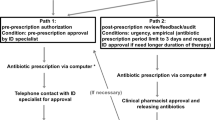
Abstract
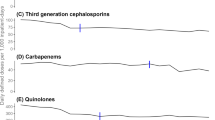
Background While various strategies for antibiotic restrictions have been validated, their impacts are not well described in smaller, non-teaching facilities. Fluoroquinolones are an appropriate target for restriction based on their propensity for overuse and potential for causing “collateral damage.” Aim Evaluate the impact of a multifaceted approach to decreasing fluoroquinolone use on consumption of fluoroquinolones and alternative antibiotics at a smaller, non-teaching facility. Method Prospective, interrupted time series analysis conducted at a single 288-bed, non-teaching hospital with 71 adult ICU beds comparing antibiotic consumption measured monthly by defined daily doses per 1000 adjusted patient days (DDD/1000 APD) prior to intervention (January 2011 to August 2014) to short-term (October 2014 to December 2015) and long-term (January 2018 to December 2019) periods following intervention. Results An increase in downward trends of fluoroquinolone use was observed from prior to intervention (-0.49 DDD/1000 APD) to the short-term period (-1.13 DDD/1000 APD) and to a greater extent in the long-term period following the intervention (-1.32 DDD/1000 APD). Fluoroquinolone consumption decreased from 100.20 DDD/1000 APD in August 2014 to 73.96 DDD/1000 APD in the short-term and 14.89 DDD/1000 APD in the long-term intervention period. Levofloxacin susceptibility for Pseudomonas aeruginosa increased from 61% to 2014 to 83% in 2018. No deleterious effects on Pseudomonas aeruginosa susceptibilities were observed for alternative antibiotics. Conclusion A multifaceted approach aimed at decreasing fluoroquinolone use at a community hospital led to a sustained decrease in consumption and a substantial increase in levofloxacin susceptibility to Pseudomonas aeruginosa.



Similar content being viewed by others
References
Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; e51-77. doi: https://doi.org/10.1093/cid/ciw118. Epub 2016 Apr 13. PMID: 27080992; PMCID: PMC5006285. Accessed June 29, 2021.
Chung GW, Wu JE, Yeo CL, et al. Antimicrobial stewardship: a review of prospective audit and feedback systems and an objective evaluation of outcomes. Virulence. 2013 Feb 15;4(2):151–7. doi: https://doi.org/10.4161/viru.21626. Epub 2013 Jan 9. PMID: 23302793; PMCID: PMC3654615.
Reed EE, Stevenson KB, West JE, et al. Impact of formulary restriction with prior authorization by an antimicrobial stewardship program. Virulence. 2013 Feb 15;4(2):158 – 62. doi: https://doi.org/10.4161/viru.21657. Epub 2012 Nov 15. PMID: 23154323; PMCID: PMC3654616.
Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Sys Rev. 2017 Feb 9;2(2):CD003543. doi: https://doi.org/10.1002/14651858.CD003543.pub4. PMID: 28178770; PMCID: PMC6464541. Accessed June 29, 2021.
Claeys KC, Hopkins TL, Vega AD, et al. Fluoroquinolone Restriction as an Effective Antimicrobial Stewardship Intervention. Curr Infect Dis Rep. 2018 Mar 23;20(5):7. doi: https://doi.org/10.1007/s11908-018-0615-z. PMID: 29572691.
Sarma JB, Marshall B, Cleeve V, et al. Effects of fluoroquinolone restriction (from 2007 to 2012) on resistance in Enterobacteriaceae: interrupted time-series analysis. J Hosp Infect. 2015 Sep;91(1):68–73. doi: 10.1016/j.jhin.2015.05.006. Epub 2015 Jun 9. PMID: 26122624.
Shea KM, Hobbs ALV, Jaso TC, et al. Effect of a Health Care System Respiratory Fluoroquinolone Restriction Program To Alter Utilization and Impact Rates of Clostridium difficile Infection. Antimicrob Agents Chemother. 2017 May 24;61(6):e00125-17. doi: https://doi.org/10.1128/AAC.00125-17. PMID: 28348151; PMCID: PMC5444144.
Lewis GJ, Fang X, Gooch M, et al. Decreased resistance of Pseudomonas aeruginosa with restriction of ciprofloxacin in a large teaching hospital’s intensive care and intermediate care units. Infect Control Hosp Epidemiol. 2012 Apr;33(4):368 – 73. doi: https://doi.org/10.1086/664763. PMID: 22418632.
Lafaurie M, Porcher R, Donay JL, et al. Reduction of fluoroquinolone use is associated with a decrease in methicillin-resistant Staphylococcus aureus and fluoroquinolone-resistant Pseudomonas aeruginosa isolation rates: a 10 year study. J Antimicrob Chemother. 2012 Apr;67(4):1010-5. doi: https://doi.org/10.1093/jac/dkr555. Epub 2012 Jan 11. PMID: 22240401.
Werner NL, Hecker MT, Sethi AK, et al. Unnecessary use of fluoroquinolone antibiotics in hospitalized patients. BMC Infect Dis. 2011 Jul 5;11:187. doi: https://doi.org/10.1186/1471-2334-11-187. PMID: 21729289; PMCID: PMC3145580.
Poole K. Multidrug efflux pumps and antimicrobial resistance in Pseudomonas aeruginosa and related organisms. J Mol Microbiol Biotechnol. 2001 Apr;3(2):255–64. PMID: 11321581.
Boel J, Andreasen V, Jarløv JO, et al. Impact of antibiotic restriction on resistance levels of Escherichia coli: a controlled interrupted time series study of a hospital-wide antibiotic stewardship programme. J Antimicrob Chemother. 2016 Jul;71(7):2047–51. doi:https://doi.org/10.1093/jac/dkw055. Epub 2016 Apr 7. PMID: 27055759.
WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment. 2021. Oslo, 2020. ISBN 978-82-8406-165-8.
Clinical and Laboratory Standards Institute (CLSI) Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data. 4th ed. CLSI; Wayne, PA, USA. 2014. Approved Guideline. M39-A4. ISBN Number: 978-1-68440-132-1.
FDA Drug Safety Communication. FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. U.S. Food and Drug Administration; 2016.
Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002 Aug;27(4):299–309. doi: https://doi.org/10.1046/j.1365-2710.2002.00430.x. PMID: 12174032.
Hunt A. FDA in Brief: FDA warns that fluoroquinolone antibiotics can cause aortic aneurysm in certain patients. U.S. Food & Drug Administration; 2018.
European Medicines Agency. Fluoroquinolone and quinolone antibiotics: PRAC recommends new restrictions on use following review of disabling and potentially long-lasting side effects. ema.europa.eu/en/news/fluoroquinolone-quinolone-antibiotics-prac-recommends-new-restrictions-use-following-review. Accessed 14.2.2022.
Medicines and Healthcare products Regulatory Agency. Drug Safety Update volume 12, issue 8. March 2019: 1.
Medicines and Healthcare products Regulatory Agency. Drug Safety Update volume 14, issue 5. December 2020: 1.
Therapeutic Goods Administration. Update - fluoroquinolone antibiotics and adverse events. tga.gov.au/publication-issue/update-fluoroquinolone-antibiotics-and-adverse-events. Accessed 14.2.2022.
Polk RE, Fox C, Mahoney A, et al. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis. 2007 Mar 1;44(5):664 – 70. doi: https://doi.org/10.1086/511640. Epub 2007 Jan 22. PMID: 17278056.
Acknowledgements
The authors would like to acknowledge the support of Laurel Golding, MA for statistical analysis and Dr. Jayesh Patel, MD who is physician champion of the Antimicrobial Management Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Belsky, B., Minson, Q. Short- and long-term impact of a multifaceted approach targeting fluoroquinolone use in a community hospital: an interrupted time-series analysis. Int J Clin Pharm 44, 741–748 (2022). https://doi.org/10.1007/s11096-022-01405-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01405-8