Abstract
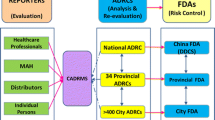
Background Rational drug use and drug safety are becoming increasingly important concerns in China with the increasing public access to drugs and the health-care system, and this has led to the development of pharmacovigilance in China. Aim of the review To provide a brief introduction about pharmacovigilance in China in terms of system development, utilization and challenges. Method Relevant studies on pharmacovigilance related to the study aim was undertaken through literature search to synthesize the extracted data. Results The creation and evolvement of China’s pharmacovigilance system spans across 30 years since 1989. The system consists of four progressing administrative layers: county, municipal, provincial and national levels. China has passed over 20 laws and regulations related to pharmacovigilance covering the processes of drug development, manufacture, distribution and use with the aim to guard drug safety. An online spontaneous self-reporting Adverse Drug Reaction (ADR) Monitoring System was established in 2003. ADRs are mainly reported by medical institutions, pharmaceutical manufacturers, and drug distributors. Currently there is no mandatory ADR reporting requirement for pharmaceutical manufacturers, and a proposed regulation under public comment will likely change this. China has started to build active pharmacovigilance surveillance programs in addition to the passive ADR reporting system. The China Food and Drug Administration has established the intensive Safety Monitoring Program and the National Adverse Drug Reaction Monitoring Sentinel Alliance Program based on electronic health records to further the efforts of ADR reporting, monitoring and analysis. Conclusion The practice of ADR monitoring and pharmacovigilance in China have made great progress. More efforts are needed both in system building, and creation of laws and regulations to strengthen the safe use of medicines.




Similar content being viewed by others
References
World Health Organization. The importance of pharmacovigilance: safety monitoring of medical products. 2002. http://apps.who.int/medicinedocs/collect/medicinedocs/pdf/s4893e/s4893e.pdf. Accessed 18 Nov 2017.
Handbook of resolutions and decisions of the World Health Assembly and Executive Board, Vol 11948-1972. Geneva: World Health Organization, 1973. WHA16.36 Clinical and pharmacological Evaluation of Drugs.
Uppsala Monitoring Centre (UMC). WHO Programme members. https://www.who-umc.org/. Accessed 22 Jun 2017.
Aagaard L, Soendergaard B, Andersen E, Kampmann JP, Hansen EH. Creating knowledge about adverse drug reactions: a critical analysis of the Danish reporting system from 1968 to 2005. Soc Sci Med. 2007;65(6):1296–309.
The International Trade Administration. Top Markets Report Pharmaceuticals Country Case Study. 2016. https://www.trade.gov/topmarkets/pdf/Pharmaceuticals_China.pdf. Accessed 28 Dec 2017.
Zhou H, Zeng F, Tang J. Pharmacovigilance in China. In: Elizabeth BA, Moore N, editors. Mann’s Pharmacovigilance. 3rd ed. New York: Wiley; 2014. p. 263–5.
The World Health Organization-Uppsala Monitoring Center. Members of the WHO Programme for International Drug Monitoring. 2017. https://www.who-umc.org/global-pharmacovigilance/members/who-programme-members/. Accessed 28 Dec 2017.
China Food and Drug Administration. Measures for the Reporting and Monitoring of Adverse Drug Reaction (Decree No.7 of Administration, P.R.China). 2004. http://www.sda.gov.cn/WS01/CL0053/24477.html. Accessed 22 Dec 2017.
China Food and Drug Administration. Measures for the Reporting and Monitoring of Adverse Drug Reaction (Decree No.84 of Ministry of Health, P. R. China). 2011. http://www.sda.gov.cn/WS01/CL0053/62621.html. Accessed 22 Dec 2017.
Zhang L, Wong LY, He Y, Wong IC. Pharmacovigilance in China: current situation, successes and challenges. Drug Saf. 2014;37(10):765–70.
China Food and Drug Administration Department of Pharmacovigilance and National Adverse Drug Reaction Monitoring Center. Handbook for Regulations on Adverse Drug Events Reporting and Monitoring. November 2012. p. 38–44.
China Food and Drug Administration Department of Pharmacovigilance and National Adverse Drug Reaction Monitoring Center. Appendix3. Assessment Criteria of Common Adverse Drug Events. Handbook for Regulations on Adverse Drug Events Reporting and Monitoring. November 2012. p. 47–48.
Biswas P. Pharmacovigilance in Asia. J Pharmacol Pharmacother. 2013;4(Suppl 1):S7.
Zhang Y, Han X, Gong S. Analysis of pharmacovigilance system of China based on a perspective of WHO pharmacovigilance system framework. Chin J Pharmacoepidemiol. 2016;25(11):725–30.
China Food and Drug Administration. Measures for the Reporting and Monitoring of Adverse Drug Reaction (for Trial Implementation). 1999. http://www.sfda.gov.cn/WS01/CL0058/9305.html. Accessed 6 Jan 2018.
Li Z, Yan J, Liu X, Ye Z, Yang X, Meyboom R, et al. Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: current status and future perspective. J Ethnopharmacol. 2012;140(3):519–25.
China Food and Drug Administration Department of Pharmacovigilance and National Adverse Drug Reaction Monitoring Center. Handbook for Regulations on Adverse Drug Events Reporting and Monitoring. November 2012. p. 45–62.
The World Health Organization-Uppsala Monitoring Center. The use of the WHO-UMC system for standardized case causality assessment. https://www.who-umc.org/media/2768/standardised-case-causality-assessment.pdf. Accessed 11 Dec 2017.
Aagaard L, Hansen EH. Information about ADRs explored by pharmacovigilance approaches: a qualitative review of studies on antibiotics. SSRIs and NSAIDs. BMC Clinical Pharmacology. 2009;9(1):4.
Beijing Food and Drug Administration. Guidelines for Monitoring of Drug Safety in 2008: Appendix1-Guidance on the Establishment of the Intensive Safety Monitoring Program (SMP). Accessed 14 Nov 2017.
China Food and Drug Administration National Center for ADR Monitoring. Research and Establishment of China Hospital Pharmacovigilance System (CHPS). 2016. http://www.cdr-adr.org.cn/dfdt/201606/t20160627_17737.html. Accessed 22 Dec 2017.
China Food and Drug Administration National Center for ADR Monitoring. Newsletter. 2017. http://www.cdr-adr.org.cn/xwdt/201712/t20171214_19834.html. Accessed 22 Dec 2017.
World Health Organization. WHO pharmacovigilance indicators: a practical manual for the assessment of pharmacovigilance systems. 2015. http://apps.who.int/iris/bitstream/10665/186642/1/9789241508254_eng.pdf. Accessed 28 Dec 2017.
China Food and Drug Administration National Center for ADR Monitoring. 2017. http://www.cdr-adr.org.cn/. Accessed 22 Dec 2017.
China Food and Drug Administration. Annual report on national adverse drug reaction monitoring (2017). http://www.sda.gov.cn/WS01/CL0844/172167.html. Accessed 23 Apr 2018.
China Food and Drug Administration. The Sixth China pharmacovigilance Conference. Report on national adverse drug reaction monitoring (2017). http://epaper.cnpharm.com/zgyyb/html/2017-09/26/content_570553.htm?div=1. Accessed 22 Dec 2017.
Aagaard L, Strandell J, Melskens L, Petersen PS, Holme HE. Global patterns of adverse drug reactions over a decade: analyses of spontaneous reports to VigiBase™. Drug Saf. 2012;35(12):1171–82.
Food and Drug Administration. FDA Adverse Event Reporting System (FAERS): Latest Quarterly Data Files. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm. Accessed 5 Jan 2017.
The European Medicines Agency (EMA). Pharmacovigilance. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000258.jsp&mid=WC0b01ac0580b18c76#. Accessed 22 Dec 2017.
China Food and Drug Administration. Seeking public comment on drug manufacturers being required to report ADRs. 2017. http://www.sda.gov.cn/WS01/CL0050/220712.html. Accessed 28 Dec 2017.
Wang T, Ma X, Xing Y, Sun S, Zhang H, Sturmer T, et al. Use of epinephrine in patients with drug-induced anaphylaxis: an analysis of the beijing pharmacovigilance database. Int Arch Allergy Immunol. 2017;173(1):51–60.
Zhao Y, Sun S, Li X, Ma X, Tang H, Sun L, et al. Drug-induced anaphylaxis in China: a 10 year retrospective analysis of the Beijing Pharmacovigilance Database. Int J Clin Pharm. 2017. https://doi.org/10.1007/s11096-017-0535-2.
Xing Y, Zhang H, Sun S, Ma X, Pleasants RA, Tang H, et al. Clinical features and treatment of pediatric patients with drug-induced anaphylaxis: a study based on pharmacovigilance data. Eur J Pediatr. 2018;177(1):1–10.
Zhao Y, Lu H, Thai S, Li X, Hui J, Tang H, et al. Development and validation of an algorithm to identify drug-induced anaphylaxis in the Beijing Pharmacovigilance Database. Int J Clin Pharm. 2018. https://doi.org/10.1007/s11096-018-0594-z.
Food and Drug Administration. Questions and Answers on FDA’s Adverse Event Reporting System (FAERS). 2017. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. Accessed 22 Dec 2017.
Cong LL, Bai YX, Hong-Yao LI, Shao ML. Comparison of pharmacovigilance information utilization in WHO, Europe, USA and China. Chin J New Drugs. 2015;24(8):844–8.
Warrer P, Hansen EH, Juhl-Jensen L, Aagaard L. Using text-mining techniques in electronic patient records to identify ADRs from medicine use. Br J Clin Pharmacol. 2012;73(5):674–84.
Chen W, Li C, Jiang J, Deng J. Research of signal detection and automatic warning technology for adverse drug reaction cased on BCPNN method. Appl Res Comput. 2009;26(4):1394–7.
Curtis LH, Weiner MG, Boudreau DM, Cooper WO, Daniel GW, Nair VP, et al. Design considerations, architecture, and use of the Mini-Sentinel distributed data system. Pharmacoepidemiology and Drug Safety. 2012;21(Suppl 1(S1)):23.
Stang PE, Ryan PB, Racoosin JA, Overhage JM, Hartzema AG, Reich C, et al. Advancing the science for active surveillance: rationale and design for the observational medical outcomes partnership. Ann Intern Med. 2010;153(9):600–6.
Acknowledgements
The authors would like to thank Matthew T. Murphy on the language editing.
Funding
This paper received no funding from any agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
All authors declare that they have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Y., Wang, T., Li, G. et al. Pharmacovigilance in China: development and challenges. Int J Clin Pharm 40, 823–831 (2018). https://doi.org/10.1007/s11096-018-0693-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-018-0693-x