Abstract
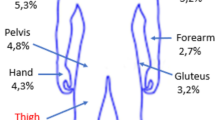
Background Treatment of Gram-positive pathogens remains a major health issue due to the presence of methicillin-resistant Staphylococcus aureus (MRSA) and Vancomycin-Resistant Enterococcus spp. Daptomycin offers an alternative after therapeutic failure using glycopeptides. Yet its use requires strict control given its financial impact and environmental risks. Since 2014, the use of daptomycin within our hospital has intensified, occasionally outside the scope of its approved indications. Objectives: The aim of this study is to analyze the appropriateness of daptomycin prescriptions. Setting This work was conducted in a 1500-bed University Hospital. Method A descriptive retrospective study was conducted from November 2013 to July 2014. All patients having received at least 2 days of treatment were included. Analysis of the appropriateness of daptomycin prescriptions was conducted by a multidisciplinary team comprised of infectious diseases specialists, pharmacists and a microbiologist. The appropriateness of daptomycin prescriptions was established based on Infectious Diseases Society of America recommendations published in 2011. Main outcome measures: The indicators chosen to determine appropriateness of prescription were: treatment indication, prescribed dose and other antibiotics associated with the daptomycin prescription. Results 19 patients (14 men/5 women) were included. Observed indications were: bone and joint infection (n = 6; 32 %), infectious endocarditis (n = 5; 26 %), bacteremia (n = 5; 26 %) and complicated skin and soft tissue infection (n = 3; 16 %). Identified pathogens were: MRSA (n = 14; 74 %), methicillin-resistant coagulase-negative Staphylococcus (n = 4; 21 %) and Streptococcus mitis (n = 1; 5 %). Daptomycin was prescribed as first-line treatment in 32 % of cases (n = 6). The mean dosage was 9 mg/kg/day (5–11 mg/kg/day) for a mean duration of 11 days (2; 55 days). Clinical success was observed in 42 % of cases (n = 8). Appropriateness for daptomycin use was only established for 15 % of prescriptions (n = 3). Conclusion Faced with a lack of recent recommendations on the subject, our multidisciplinary team issued a local consensus, defining the indications and dosage modalities for this reserve antibiotic. This multidisciplinary approach enables improved use of recent anti-MRSA drugs
Similar content being viewed by others
References
Lushniak BD. Antibiotic resistance: a public health crisis. Public Health Rep. 2014;129(4):314–6.
Enoch DA, Bygott JM, Daly ML, Karas JA. Daptomycin. J Infect. 2007;55(3):205–13.
French National Agency for Drug and Health Safety (France). Transparency Committee: daptomycin. Haute Autorité de santé; 2006 Oct. France. http://www.hassante.fr/portail/upload/docs/application/pdf/2009-01/ct_2927_cubicine_ang_2009-01-15_11-30-23_417.pdf. Accessed Feb 2014
Arbeit RD, Maki D, Tally FP, Capanaro E, Eisenstein BI. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis. 2004;38(12):1673–81.
Quist SR, Fierlbeck G, Seaton RA, Loeffler J, Chaves RL. Comparative randomized clinical trial against glycopeptides supports the uses of daptomycin as first-line treatment of complicated skin and soft-tissue infections. Int J Antimicrob Agents. 2012;39(1):90–1.
Fowler VG Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653–65.
Boucher HW, Sakoulas G. Perspectives on daptomycin resistance with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis. 2007;45(5):601–8.
Van Hal SJ, Paterson L, Gosbell IB. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteremia in a daptomycin naïve patient, a review of the literature. Eur J Clin Microbiol Dis. 2011;30(5):603–10.
Humphries RM, Pollett S, Saoulais G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev. 2013;26(4):759–80.
Cui L, Tominaga E, Neoh HM, Himaratsu K. Correlation between reduced daptomycin susceptibility and vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50(3):1079–82.
Tenover FC, Sinner SW, Segal RE, Huang V, Alexandre SS, McGowan JE Jr, et al. Characterization of a Staphylococcus aureus strain with progressive loss of susceptibility to vancomycin and daptomycin during therapy. Int J Antimicrob Agents. 2009;33(6):564–8.
Sauermann R, Rothenburger M, Graninger W, Joukhadar C. Daptomycin: a review 4 years after first approval. Pharmacology. 2008;81(2):79–91.
He W, Zhang Y, Chen H, Zhao C, Wang H. Efficacy and safety of daptomycin for the treatment of infectious disease: a meta-analysis based on randomized trials. J Antimicrob Chemother. 2014;69(12):3181–9.
Jones RN, Barry AL. Antimicrobial activity and spectrum of LY146032, a lipopeptide antibiotic, including susceptibility testing recommendations. Antimicrob Agents Chemother. 1987;31(4):625–9.
Murray KP, Zhao JJ, Davis SL, Kullar R, Kaye KS, Lephart P, et al. Early use of daptomycin versus vancomycin for methicillin-resistant Staphylococcus aureus bacteremia with vancomycin minimum inhibitory concentration >1 mg/L: a matched cohort study. Clin Infect Dis. 2013;56(11):1562–9.
Montange D, Berthier F, Leclerc G, Serre A, Jeunet L, Berard M, et al. Penetration of daptomycin into bone and synovial fluid in joint replacement. Antimicrob Agents Chemother. 2014;58(7):3991–6.
French National Agency for Drug and Health Safety (France). Clinical Practice Guidelines: Antibiotic therapy and prevention of bacterial resistance in healthcare organization. Haute Autorité de Santé; 2008 Apr. France. http://www.has-sante.fr/portail/upload/docs/application/pdf/2010-03/antibiotic_therapy_and_prevention_of_bacterial_resistance_guidelines.pdf. Accessed March 2014
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):285–92.
French National Agency for Drug and Health Safety (France). Caractérisation des antibiotiques considérés comme « critiques». ANSM. 2013 Nov. France. http://ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Les-antibiotiques-consideres-comme-critiques-premieres-reflexions-sur-leur-caracterisation-Point-d-information. Accessed March 2014
Selçuk K, Gürdal Y, Ahmet K, Beriç E, Iftihar K. Treatment of Gram positive left sided infective endocarditis with daptomycin. J Infect Chemother. 2013;19(4):698–702.
Kullar R, Casapao AM, Davis SL, Levine DP, Zhao JJ, Crank CW, et al. A multicentre evaluation of the effectiveness and safety of high-dose daptomycin for the treatment of infective endocarditis. J Antimicrob Chemother. 2013;68(12):2921–6.
Sakoulas G, Alder J, Thauvin-Eliopoulos C, Moellering RC Jr, Eliopoulos GM. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob Agents Chemother. 2006;50(4):1581–5.
Lamp KC, Friedrich LV, Mendez-Vigo L, Russo R. Clinical experience with daptomycin for the treatment of patients with osteomyelitis. Am J Med. 2007;120(10):S13–20.
Byren I, Rege S, Campanaro E, Yankelev S, Anastasiou D, Kuropatkin G, et al. Randomized controlled trial ofthe safety and efficacy of daptomycin versus standard-of-care therapy for management of patients with osteomyelitis associated with prosthetic devices undergoing two-stage revision arthroplasty. Antimicrob Agents Chemother. 2012;56(11):5626–32.
Pablo S, Perez-Cardona C, Barro Ojeda V, Rodriguez Pardo D, Pigrau Serrallach C. Clinical experience with daptomycin for the treatment of patients with knee and hip periprosthetic joint infections. J Antimicrob Chemother. 2012;67(7):1749–54.
Liang SL, Khair HN, McDonald JR, Badcock HM, Marschall J. Daptomycin versus vancomycin for osteoarticular infections due to methicillin-resistant Staphylococcus aureus (MRSA): a nested case-control study. Eur J Clin Microbiol Infect Dis. 2014;33(4):659–64.
Basseti M, Nicco E, Ginocchio F, Ansaldi F, De Florentiis D, Viscoli C. High-dose daptomycine in documented Staphylococcus aureus infections. Int J Antimicrob Agents. 2010;36(5):459–61.
Dohmen PM, Guleri A, Capone A, Utili R, Seaton RA, González-Ramallo VJ, et al. Daptomycin for the treatment of infective endocarditis: results from a European registry. J Antimicrob Chemother. 2013;68(4):936–42.
Dellit TH, Owens RC, McGowan JE Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77.
Acknowledgments
The authors thank the following pharmacists for their contribution to data collection: Morgane Bonnet, Angélique Cotteau-Leroy, Marie-Christine Douet, Thierry Henon, Dominique Levêque, Isabelle Poujol and Isabelle Tiret.
Funding
The authors received no funding for this study.
Conflicts of interest
The authors declare they have no conflict of interest concerning this article
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castel, C., de La Blanchardière, A., Michon, J. et al. Study on daptomycin use and implementation of an antimicrobial stewardship program. Int J Clin Pharm 38, 421–428 (2016). https://doi.org/10.1007/s11096-016-0271-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-016-0271-z