Abstract
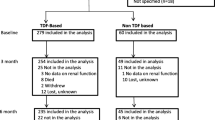
Background Since the beginning of highly active antiretroviral therapy utilization, the association of renal impairment with treatment toxicity is more prevalent. Tenofovir disoproxil fumarate (TDF) side effects include renal toxicity. Objective To assess the incidence of renal damage in human immunodeficiency virus (HIV)-positive patients treated with TDF and to identify associated potential risk factors. Setting A public university tertiary 450-beds hospital in Spain. Method Retrospective, longitudinal observational study that included adult HIV-1-infected patients treated with TDF. Patient´s treated with TDF from January 2010 to December 2012 were included. Patient follow-up started when initiating treatment with TDF up until either end of treatment or end of study (July 31, 2013). The estimated glomerular filtration rate was calculated using the four-variable modification of diet in renal disease. Renal toxicity was classified as moderate [estimated glomerular filtration rate (eGFR) < 60 ml/min] or severe (eGFR < 30 ml/min). The incidence rate for moderate and severe renal insufficiency was calculated as number of cases per 1000 patient-year. A univariate analysis and binary logistic regression was carried out in order to identify risk factors associated with renal toxicity by using the forward stepwise method (likelihood ratio) Main outcome measure: Incidence rate for moderate and severe renal insufficiency (RI) Results 451 patients were included in the study. The incidence rate of moderate RI was 29.2 cases per 1000 person-year (95 % CI 22.1–36.3), whereas the incidence of severe RI was 2.2 cases per 1000 person-year (95 % CI 0.3–4.1). Multivariate analysis confirmed an independent association with the risk of kidney damage for age (OR 1.08 95 % CI 1.05–1.12), time on treatment with TDF (OR 1.16 95 % CI 1.04–1.30), baseline creatinine (OR 49.80 95 % CI 7.90–311.92) and treatment with NNRTIs (OR 0.45 95 % CI 0.24–0.83). Conclusion Mild to moderate renal failure is a frequent complication during treatment with TDF although severe renal impairment is scarce. Risk factors include age, duration of treatment with TDF, elevated baseline creatinine levels, and treatment with protease inhibitor boosted with ritonavir combinations.
Similar content being viewed by others
References
Kilmarx PH. Global epidemiology of HIV. Curr Opin HIV AIDS. 2009;4(4):240–6.
Lee SH, Kim KH, Lee SG, Chen DH, Jung DS, Moon CS, et al. Trends of mortality and cause of death among HIV-infected patients in Korea, 1990–2011. J Korean Med Sci. 2013;28(1):67–73.
Chowers MY, Gottesman BS, Leibovici L, Pielmeier U, Andreassen S, Paul M. Reporting of adverse events in randomized controlled trials of highly active antiretroviral therapy: systematic review. J Antimicrob Chemother. 2009;64(2):239–50.
Heath KV, Singer J, O’Shaughnessy MV, Montaner JSG, Hogg RS. Intentional nonadherence due to adverse symptoms associated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(2):211–7.
d’Arminio Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, et al. Insights into the reasons for discontinuation of the first highly active antirretroviral therapy (HAART) regimen in a cohort of antirretroviral naïve patients. AIDS. 2000;14(5):499–507.
Ammassari A, Murri R, Pezzotti P, Trotta MP, Ravasio L, De Longis P, et al. Self reported symptoms and medications side effects influence adherence to highly active antiretroviral therapy in persons with HIV infection. J Acquir Immune Defic Syndr. 2001;28(5):445–9.
Martín MT, del Cacho E, López E, Codina C, Tuset M, de Lazzari E, et al. Adverse side effects of antiretroviral therapy: relationship between patients’ perception and adherence. Med Clin (Barc). 2007;129(4):127–33.
Schwartz EJ, Szczech LA, Ross MJ, Klotman ME, Winston JA, Klotman PE. HAART and the epidemic of HIV end stage renal disease. J Am Soc Nephrol. 2005;16:2412–20.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents [Internet]. Department of Health and Human Services; 2014 [cited 2014 May 22]. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf.
Viread® EPAR_Product Information [Internet]. European Medicines Agency; 2014 [cited 2014 Mar 17]. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/000419/WC500051737.pdf.
Shepp DH, Curtis S, Rooney JF. Causes and consequences of hypokalemia in patients on tenofovir disoproxil fumarate. AIDS. 2007;21(11):1479–81.
Jafari A, Khalili H, Dashti-Khavidaki S. Tenofovir-induced nephrotoxicity: incidence, mechanism, risk factors, prognosis and proposed agents for prevention. Eur J Clin Pharmacol. 2014;70(9):1029–40.
Calza L, Trapani F, Salvadori C, Magistrelli E, Manfredi R, Colangeli V, et al. Incidence of renal toxicity in HIV-infected, antiretroviral-naïve patients starting tenofovir/emtricitabine associated with efavirenz, atazanavir/ritonavir, or lopinavir/ritonavir. Scand J Infect Dis. 2013;45(2):147–54.
Bonjoch A, Echeverría P, Perez-Alvarez N, Puig J, Estany C, Clotet B, et al. High rate of reversibility of renal damage in a cohort of HIV-infected patients receiving tenofovir-containing antiretroviral therapy. Antiviral Res. 2012;96(1):65–9.
Lai S, Mariotti A, Lai C, Testorio M, Carta M, Innico G et al. Tenofovir-Related Nephropathies in HIV-Infected Patients. Curr Vasc Pharmacol. 2014 [Epub ahead of print].
Monteiro N, Branco M, Peres S, Borges F, Mansinho K. The impact of tenofovir disoproxil fumarate on kidney function: four-year data from the HIV-infected outpatient cohort. J Int AIDS Soc. 2014;17(4 Suppl 3):19565.
Woratanarat K, Kanjanabuch T, Suankratay C. Tenofovir disoproxil fumarate-associated nephrotoxicity in HIV-infected patients: a prospective controlled study. J Med Assoc Thai. 2013;96(4):432–9.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation; classification; and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266.
Rodriguez-Nóvoa S, Alvarez E, Labarga P, Soriano V. Renal toxicity associated with tenofovir use. Expert Opin Drug Saf. 2010;9(4):545–59.
Ganesan A, Krantz EM, Huppler Hullsiek K, Riddle MS, Weintrob AC, Lalani T, et al. Determinants of incident chronic kidney disease and progression in a cohort of HIV-infected persons with unrestricted access to health care. HIV Med. 2013;14(2):65–76.
Scherzer R, Estrella M, Li Y, Deeks SG, Grunfeld C, Schlipak MG. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26(7):867–75.
Monteagudo-Chu MO, Chang MH, Fung HB, Bräu N. Renal toxicity of long-term therapy with tenofovir in HIV-infected patients. J Pharm Pract. 2012;25(5):552–9.
Madeddu G, Bonfanti P, De Socio GV, Carradori S, Grosso C, Marconi P, et al. Tenofovir renal safety in HIV-infected patients: results from the SCOLT Project. Biomed Pharmacother. 2008;62(1):6–11.
Mauss S, Berger F, Schmutz G. Antiretroviral therapy with tenofovir is associated with mild renal dysfunction. AIDS. 2005;19(1):93–5.
Wikman P, Safont P, Palacio M, Moreno A, Moreno S, Casado JL. The significance of antiretroviral-associated acute kidney injury in a cohort of ambulatory human immunodeficiency virus-infected patients. Nephrol Dial Transplant. 2013;28(8):2073–81.
Post FA, Campbell LJ, Hamzah L, Collins L, Jones R, Siwani R, et al. Predictors of renal outcome in HIV-associated nephropathy. Clin Infect Dis. 2008;46(8):1282–9.
Roe J, Campbell LJ, Ibrahim F, Hendry BM, Post FA. HIV care and the incidence of acute renal failure. Clin Infect Dis. 2008;47(2):242–9.
Ibrahim F, Hamzah L, Jones R, Nitsch D, Sabin C, Post F. Baseline kidney function as predictor of mortality and kidney disease progression in HIV-positive patients. Am J Kidney Dis. 2012;60(4):539–47.
Mulenga LB, Kruse G, Lakhi S, Cantrell RA, Reid SE, Zulu I, et al. Baseline renal insufficiency and risk of death among HIV-infected adults on antiretroviral therapy in Lusaka, Zambia. AIDS. 2008;22(14):1821–7.
Estrella MM, Parekh RS, Abraham A, Astor BC, Szczech LA, Anastos K, et al. The impact of kidney function at highly active antiretroviral therapy initiation on mortality in HIV-infected women. J Acquir Immune Defic Syndr. 2010;55(2):217–20.
Choi A, Scherzer R, Bacchetti P, Tien PC, Saag MS, Gibert CL, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010;56(5):872–82.
Goicoechea M, Liu S, Best B, Sun S, Jain S, Kemper C, et al. Greater tenofovir associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008;197(1):102–8.
Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr. 2006;43(3):278–83.
Kiser JJ, Carten ML, Aquilante CL, Anderson PL, Wolfe P, King TM, et al. Effect of lopinavir/ritonavir on the renal clearance of tenofovir in HIV-infected patients. Clin Pharmacol Ther. 2008;83(2):265–72.
Jullien V, Treluyer JM, Rey E, Jaffray P, Krivine A, Moachon L, et al. Population pharmacokinetics of tenofovir in human immunodeficiency virus-infected patients taking highly active antiretroviral therapy. Antimicrob Agents Chemother. 2005;49(8):3361–6.
Kapadia J, Shah S, Desai C, Desai M, Patel S, Shah AN, et al. Tenofovir induced Fanconi syndrome: a possible pharmacokinetic interaction. Indian J Pharmacol. 2013;45(2):191–2.
Izzedine H, Hulot JS, Villard E, Goyenvalle C, Dominguez S, Ghosn J, et al. Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis. 2006;194(11):1481–91.
Rodríguez-Novoa S, Labarga P, Soriano V, Egan D, Albalater M, Morello J, et al. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis. 2009;48(11):e108–16.
Rodríguez-Novoa S, Labarga P, D’Avolio A, Barreiro P, Albalate M, Vispo E. Impairment in kidney tubular function in patients receiving tenofovir is associated with higher tenofovir plasma concentrations. AIDS. 2010;24(7):1064–6.
Fafin C, Pugliese P, Durant J, Mondain V, Rahelinirina V, De Salvador F, et al. Increased time exposure to tenofovir is associated with a greater decrease in estimated glomerular filtration rate in HIV patients with kidney function of less than 60 ml/min/1,73 m2. Nephron Clin Pract. 2012;120(4):c205–14.
Jülg BD, Bogner JR, Crispin A, Goebel FD. Progression of renal impairment under therapy with tenofovir. AIDS. 2005;19(12):1332–3.
Nelson M, Katlama C, Montaner J, Cooper D, Gazzard B, Clotet B, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21(10):1273–81.
Pradat P, Le Pogam MA, Okon JB, Trolliet P, Miailhes P, Brochier C, et al. Evolution of glomerular filtration rate in HIV-infected; HIV-HBV-coinfected and HBV-infected patients receiving tenofovir disoproxil fumarate. J Viral Hepat. 2013;20(9):650–7.
Di Biagio A, Rosso R, Vitale F, Cardinale F, Sormani MP, Secondo G, et al. Risk factors for chronic kidney disease among human immunodeficiency virus-infected patients: a European case control study. Clin Nephrol. 2011;75(6):518–23.
Mallants R, Van Oosterwyck K, Van Vaeck L, Mols R, De Clercq E, Augustijins P. Multidrug resistance-associated protein 2 (MRP2) affects hepatobiliary elimination but not the intestinal disposition of tenofovir disoproxil fumarate and its metabolites. Xenobiotica. 2005;35(10–11):1055–66.
Miller D. Nucleoside phosphonate interactions with multiple organic anion transporters in renal proximal tubule. J Pharmacol Exp Ther. 2001;299(2):567–74.
Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis. 2005;45(5):804–17.
Funding
The authors state that they did not receive any financial reward to conduct this research. This study was conducted in a clinic environment by independent researchers.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quesada, P.R., Esteban, L.L., García, J.R. et al. Incidence and risk factors for tenofovir-associated renal toxicity in HIV-infected patients. Int J Clin Pharm 37, 865–872 (2015). https://doi.org/10.1007/s11096-015-0132-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-015-0132-1