Abstract
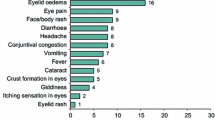
Background Statins are a class of medication indicated for atherosclerotic diseases and dyslipidemia. Since their appearance, many adverse events have been associated with their use. Ocular disorders are rare but serious adverse events of statins. Objective To report the association between statins and ocular adverse events (blurred vision, visual impairment, visual field defect, reduced visual acuity, myopia, hypermetropia, presbyopia, and astigmatism) which might be associated with muscle or liver problems by examining the frequency of ocular adverse events among the reported adverse drug reactions from the Food and Drug Administration (FDA) and Adverse Drug Reactions Advisory Committee (ADRAC) data. Setting The FDA USA and ADRAC Australia databases. Methods We conducted a retrospective study of statin-associated ocular adverse events reported to FDA between 1988 and 2013 and ADRAC between 1988 and 2011. The recoded data included: patient’s age, gender, suspected drug and dosage, concomitant drug, adverse events, duration of therapy, dechallenge and rechallenge therapy. The differences in the adverse events profiles between each of the statins and atorvastatin were performed using Chi square and multivariate (logistic regression) statistical tests. Main outcome measure Percentages of subjects correlated with each Ocular adverse events. Results Among 131,755 cases of patients taking statins in the FDA, there were 2325 cases reported ocular adverse events after using statins (1.8 %). The Chi square statistic showed that the proportions of ocular adverse events varied significantly (p < 0.0001) across the different statin drugs. The most highly reported ocular adverse events associated with statins were blurred vision (48.4 %) and visual impairment (25.7 %). Results from logistic regression indicated that the ocular problems formed a greater proportion of the adverse events for subjects taking atorvastatin (2.1 %). Of the 1.8 %, ocular adverse events mostly occurred alone (60.9 %), followed by 30.3 % where muscle adverse events also were involved. The ADRAC data held 136 cases of statins associated ocular adverse events (47 patients reported blurred vision and 64 reported vision impairment). Conclusion All statins were associated with ocular side effects, with atorvastatin showed a higher incidence of ocular side effects in conjunction with muscle and liver problems.
Similar content being viewed by others
References
Navarese EP, Kowalewski M, Andreotti F, van Wely M, Camaro C, Kolodziejczak M, et al. Meta-analysis of time-related benefits of statin therapy in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Am J Cardiol. 2014;113(10):1753–64.
Sirtori CR. The pharmacology of statins. Pharmacol Res. 2014;88:3–11.
Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–90.
Hedenmalm K, Alvan G, Ohagen P, Dahl M-L. Muscle toxicity with statins. Pharmacoepidemiol Drug Saf. 2010;19:223–31.
Fraunfelder FW, Richards AB. Diplopia, blepharoptosis, and ophthalmoplegia and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor use. Ophthalmology. 2008;115:2282–5.
Vitale S, Ellwein L, Cotch MF, Ferris FL 3rd, Sperduto R. Prevalence of refractive error in the United States, 1999–2004. Arch Ophthalmol. 2008;126:1111–9.
Fraunfelder FW. Ocular adverse drug reactions. Expert Opin Drug Saf. 2003;2:411–20.
Oh SJ, Dhall R, Young A, Morgan MB, Lu L, Claussen GC. Statins may aggravate myasthenia gravis. Muscle Nerve. 2008;38:1101–7.
Irimia P, Moya M, Sepulcre J, Martinez-Vila E. Transient blurred vision as the only manifestation of basilar stenosis. Cerebrovasc Dis. 2004;18:88–9.
Negevesky GJ, Kolsky MP, Laureno R, Yau TH. Reversible atorvastatin-associated external ophthalmoplegia, anti-acetylcholine receptor antibodies, and ataxia. Arch Ophthalmol. 2000;118:427–8.
Sasaki J, Ikeda Y, Kuribayashi T, Kajiwara K, Biro S, Yamamoto K, et al. A 52-week, randomized, open-label, parallel-group comparison of the tolerability and effects of pitavastatin and atorvastatin on high-density lipoprotein cholesterol levels and glucose metabolism in Japanese patients with elevated levels of low-density lipoprotein cholesterol and glucose intolerance. Clin Ther. 2008;30:1089–101.
American Academy of Ophthalmology. [cited 1 August 2014]. http://www.aao.org/.
Beltowski J, Wojcicka G, Jamroz-Wisniewska A. Adverse effects of statins—mechanisms and consequences. Curr Drug Saf. 2009;4:209–28.
Eckel RH. Approach to the patient who is intolerant of statin therapy. J Clin Endocrinol Metab. 2010;95:2015–22.
Fraunfelder FW. Ocular hemorrhage possibly the result of HMG-CoA reductase inhibitors. J Ocul Pharmacol Ther. 2004;20:179–82.
Baker SK, Samjoo IA. A neuromuscular approach to statin-related myotoxicity. Can J Neurol Sci. 2008;35:8–21.
Athyros VG, Kakafika AI, Tziomalos K, Karagiannis A, Mikhailidis DP. Pleiotropic effects of statins-clinical evidence. Curr Pharm Des. 2009;15:479–89.
Ertas FS, Ertas NM, Gulec S, Atmaca Y, Tanju S, Sener C, et al. Unrecognized side effect of statin treatment: unilateral blepharoptosis. Ophthalmic Plast Reconstr Surg. 2006;22:222–4.
MIMS Australia 2014, ‘Statin’, MIMS Online, Sydney, viewed 25 August 2014. http://www.mimsonline.com.au./Search/Search.aspx.
World Health Organization. Glossary of terms used in Pharmacovigilance. 2015 [cited 1 April 2015]. who-umc.org/Graphics/24729.pdf.
Funding
This research received no specific Grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizranita, V., Pratisto, E.H. Statin-associated ocular disorders: the FDA and ADRAC data. Int J Clin Pharm 37, 844–850 (2015). https://doi.org/10.1007/s11096-015-0128-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-015-0128-x