Abstract
Background
Research on the benefits of clinical medication reviews (CMRs) performed by pharmacists has been conducted mostly in controlled settings and has been widely published. Less is known of the effects after large scale implementation in community pharmacies. An online CMR tool enabled the systematic registration of drug-related problems (DRPs) and implemented interventions derived from CMRs in daily practice.
Objective
To describe the effects of CMRs on pharmacy practice after large-scale implementation in the Netherlands.
Setting
268 community pharmacies. Pharmacists were trained on CMRs with a patient centred approach.
Method
Retrospective analyses of DRPs, pharmacists’ proposals and implemented interventions recorded between January 1st and September 1st 2012.
Main outcome measure
Frequencies of DRPs, intervention proposals, implemented interventions, and drugs involved.
Results
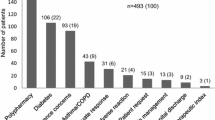
4,579 CMRs were analysed. On average 2.9 (SD 2.1) DRPs per review were identified. 4,123 (31 %) of the DRPs led to medication changes. Stopping a drug (16 %) was more frequent than starting a drug (8.1 %). Drugs related to cardiovascular risk management, diabetes and osteoporosis were most frequently involved.
Conclusion
This study is the largest analysis of pharmacists-initiated CMRs in the Netherlands to date. The findings demonstrate the potential to reduce medication-related errors through pharmacist involvements in complex pharmacotherapy and the positive impact on the quality of drug therapy through making necessary medication changes. The data also support the need for large-scale implementation of pharmacists-initiated CMRs in the presence of proper training programmes.


Similar content being viewed by others
References
FIP Working Group on Collaborative Practice. FIP reference paper collaborative practice. Den Haag: International Pharmaceutical Federation; 2009. http://www.fip.org/www/uploads/database_file.php?id=319&table_id=; accessed at: 9-12-2013.
Expertgroep Medicatieveiligheid [Expert Group Medication Safety]. HARM-WRESTLING. Den Haag: Ministerie van Volksgezondheid, Welzijn en Sport [Ministry of Health, Welfare and Sport]; 2009. http://www.knmp.nl/downloads/medicijnen-zorgverlening/medicatieveiligheid/harmwrestlingrapportdefnov2009.pdf. Accessed at: 9 dec 2013.
Patterson S, Hughes C, Kerse N, Cardwell C, Bradley M. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2012;5:CD008165.
Task Force on Medicines Partnership and The National Collaborative Medicines Management Services Programme. Room for review. A guide to medication review: the agenda for patients, practitioners and managers. 1st ed. London: Medicines Partnership; 2002. ISBN 0-9544028-0-4.
Sorensen L, Stokes J, Purdie D, Woodward M, Elliott R, Roberts M. Medication reviews in the community: results of a randomized, controlled effectiveness trial. Br J Clin Pharmacol. 2004;58(6):648–64.
Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ. 2001;323(7325):1340–3.
Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ. 2005;330(7486):293.
Geurts MME, Talsma J, Brouwers JRBJ, de Gier J. Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: a systematic review. Br J Clin Pharmacol. 2012;74(1):16–33.
Fiß T, Meinke-Franze C, van den Berg N, Hoffmann W. Effects of a three party healthcare network on the incidence levels of drug related problems. Int J Clin Pharm. 2013;35(5):763–71.
Milos V, Rekman E, Bondesson Å, Eriksson T, Jakobsson U, Westerlund T, et al. Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: a randomised controlled study. Drugs Aging. 2013;30(4):235–46.
Nederlands Huisartsen Genootschap ism andere beroepsorganisaties/instanties/verenigingen [Dutch College of General Practitioners in collaboration with other professional organisations]. Multidisciplinaire Richtlijn Polyfarmacie bij ouderen, 2012 [Multidisciplinary Guideline Polypharmacy in Elderly]. NHG 2012. https://www.nhg.org/sites/default/files/content/nhg_org/uploads/polyfarmacie_bij_ouderen.pdf; accessed at: 9-12-2013.
Denneboom W, Dautzenberg MGH, Grol R, De Smet PAGM. Treatment reviews of older people on polypharmacy in primary care: cluster controlled trial comparing two approaches. Br J Gen Pract. 2007;57(542):723–31.
Leikola SNS, Virolainen J, Tuomainen L, Tuominen R, Airaksinen MSA. Comprehensive medication reviews for elderly patients: findings and recommendations to physicians. J Am Pharm Assoc. 2012;52(5):630–3.
Vinks THAM, Egberts TCG, de Lange T, de Koning FHP. Pharmacist-based medication review reduces potential drug-related problems in the elderly: the SMOG controlled trial. Drugs Aging. 2009;26(2):123–33.
Brulhart M, Wermeille J. Multidisciplinary medication review: evaluation of a pharmaceutical care model for nursing homes. Int J Clin Pharm. 2011;33(3):549–57.
Stafford A, Tenni P, Peterson G, Jackson S, Hejlesen A, Villesen C, et al. Drug-related problems identified in medication reviews by Australian pharmacists. Pharm World Sci. 2009;31(2):216–23.
Shimp L, Kucukarslan S, Elder J, Remington T, Wells T, Choe H, et al. Employer-based patient-centered medication therapy management program: evidence and recommendations for future programs. J Am Pharm Assoc. 2012;52(6):768–76.
Nishtala P, McLachlan A, Bell JS, Chen T. A retrospective study of drug-related problems in Australian aged care homes: medication reviews involving pharmacists and general practitioners. J Eval Clin Pract. 2011;17(1):97–103.
Freeman C, Cottrell WN, Kyle G, Williams I, Nissen L. An evaluation of medication review reports across different settings. Int J Clin Pharm. 2013;35(1):5–13.
Kwint HF, Faber A, Gussekloo J, Bouvy ML. The contribution of patient interviews to the identification of drug-related problems in home medication review. J Clin Pharm Ther. 2012;37(6):674–80.
Stichting Farmaceutische Kengetallen [Foundation for Pharmaceutical Statistics]. Data en feiten 2013 [Data and facts 2013]. Den Haag: SFK; 2012. ISBN 978-90-817780-1-5.
Gezondheidsraad [Health Council of the Netherlands]. Evaluatie van de voedingsnormen voor vitamine D [Evaluation of the dietary reference values for vitamin D]. Den Haag: Gezondheidsraad; 2012. 150 p. Report No.: 2012/15. ISBN 978-90-5549-931-1.
Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–25.
Acknowledgments
The authors would like to thank all pharmacists for their efforts in recording all outcomes in the SAMRT. Also, we would like to thank all the staff from Stevenshof Institute for Research, Utrecht University and Service Apotheek who have contributed to this study. Finally, we would like to thank David Preece for his valuable input as a native speaker.
Funding
The study was funded by Nederlandse Service Apotheek Beheer BV (SA).
Conflicts of interest
PH was employed by SA. SA provided the raw data and objective information regarding the SAMRT, but both PH and SA were not involved in the selection and the interpretation of the results for this manuscript. Other authors have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kempen, T.G.H., van de Steeg-van Gompel, C.H.P.A., Hoogland, P. et al. Large scale implementation of clinical medication reviews in Dutch community pharmacies: drug-related problems and interventions. Int J Clin Pharm 36, 630–635 (2014). https://doi.org/10.1007/s11096-014-9947-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-014-9947-4