Abstract
Aims
To develop a semi-mechanistic hepatic compartmental model to predict the effects of rifampicin, a known inducer of CYP3A4 enzyme, on the metabolism of five drugs, in the hope of informing dose adjustments to avoid potential drug-drug interactions.
Methods
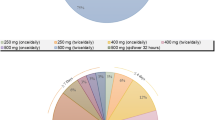
A search was conducted for DDI studies on the interactions between rifampicin and CYP substrates that met specific criteria, including the availability of plasma concentration–time profiles, physical and absorption parameters, pharmacokinetic parameters, and the use of healthy subjects at therapeutic doses. The semi-mechanistic model utilized in this study was improved from its predecessors, incorporating additional parameters such as population data (specifically for Chinese and Caucasians), virtual individuals, gender distribution, age range, dosing time points, and coefficients of variation.
Results
Optimal parameters were identified for our semi-mechanistic model by validating it with clinical data, resulting in a maximum difference of approximately 2-fold between simulated and observed values. PK data of healthy subjects were used for most CYP3A4 substrates, except for gilteritinib, which showed no significant difference between patients and healthy subjects. Dose adjustment of gilteritinib co-administered with rifampicin required a 3-fold increase of the initial dose, while other substrates were further tuned to achieve the desired drug exposure.
Conclusions
The pharmacokinetic parameters AUCR and CmaxR of drugs metabolized by CYP3A4, when influenced by Rifampicin, were predicted by the semi-mechanistic model to be approximately twice the empirically observed values, which suggests that the semi-mechanistic model was able to reasonably simulate the effect. The doses of four drugs adjusted via simulation to reduce rifampicin interaction.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Peltoniemi MA, Saari TI, Hagelberg NM, Laine K, Kurkinen KJ, Neuvonen PJ, Olkkola KT. Rifampicin has a profound effect on the pharmacokinetics of oral S-ketamine and less on intravenous S-ketamine. Basic Clin Pharmacol Toxicol. 2012;111(5):325–32.
Li X, Wu K, Zhou Z, Jia R, Zhao Y, Li J, et al. Predicting fluconazole drug-drug interactions using a physiologically based pharmacokinetic (PBPK) model. J Infect Dis Ther. 2022;10(4):1000504. Availability at: https://www.omicsonline.org/open-access-pdfs/predicting-fluconazole-drugdrug-interactions-using-a-physiologicallybased-pharmacokinetic-pbpk-model.pdf
Zhang F, Zhou Y, Wu N, Jia R, Liu A, Liu B, Zhou Z, Hu H, Han Z, Ye X. In silico prediction of bioequivalence of Isosorbide Mononitrate tablets with different dissolution profiles using PBPK modeling and simulation. Eur J Pharm Sci. 2021;157:0928–87.
Kato M, Shitara Y, Sato H, Yoshisue K, Hirano M, Ikeda T, Sugiyama Y. The quantitative prediction of CYP-mediated drug interaction by physiologically based pharmacokinetic modeling. Pharm Res. 2008;25(8):1891–901.
Kato M, Chiba K, Horikawa M, Sugiyama Y. The quantitative prediction of in vivo enzyme-induction caused by drug exposure from in vitro information on human hepatocytes. Drug Metab Pharmacokinet. 2005;20(4):236–43.
Jones H, Rowland-Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT: Pharmacometrics & Systems Pharmacology. 2013;2(8):1–12.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Poulin P, Theil FP. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism‐based prediction of volume of distribution. Journal of Pharmaceutical Sciences. 2002;91(1):129–56.
Acocella G. Pharmacokinetics and metabolism of rifampin in humans. Reviews of Infectious Diseases. 1983;5(Supplement_3):S428–32.
Chen J, Xu H, Pawlak S, James LP, Peltz G, Lee K, Ginman K, Bergeron M, Pithavala YK. The effect of rifampin on the pharmacokinetics and safety of lorlatinib: results of a phase one, open-label, crossover study in healthy participants. Adv Ther. 2020;37(2):745–58.
James AJ, Smith CC, Litzow M, Perl AE, Altman JK, Shepard D, Kadokura T, Souda K, Patton M, Lu Z, Liu C, Moy S, Levis MJ, Bahceci E. Pharmacokinetic Profile of Gilteritinib: A Novel FLT-3 Tyrosine Kinase Inhibitor. Clin Pharmacokinet. 2020;59(10):1273–90.
Mukai M, Uchimura T, Zhang X, Greene D, Vergeire M, Cantillon M. Effects of rifampin on the pharmacokinetics of a single dose of istradefylline in healthy subjects. J Clin Pharmacol. 2018;58(2):193–201.
Nguyen L, Holland J, Miles D, Engel C, Benrimoh N, O’Reilly T, Lacy S. Pharmacokinetic (PK) drug interaction studies of cabozantinib: effect of CYP3A inducer rifampin and inhibitor ketoconazole on cabozantinib plasma PK and effect of cabozantinib on CYP2C8 probe substrate rosiglitazone plasma PK. J Clin Pharmacol. 2015;55(9):1012–23.
Loos U, Musch E, Jensen J, Mikus G, Schwabe H, Eichelbaum M. Pharmacokinetics of oral and intravenous rifampicin during chronic administration. Klin Wochenschr. 1985;63(23):1205–11.
US Food and Drug Administration. Label of LORBRENA® (Lorlatinib) Tablets. 2021. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210868s004lbl.pdf. Accessed 13 Mar 2024
Gerner B, Scherf-Clavel O. Physiologically based pharmacokinetic modelling of cabozantinib to simulate enterohepatic recirculation, drug–drug interaction with rifampin and liver impairment. Pharmaceutics. 2021;13(6):778–96.
US Food and Drug Administration. Clinical Pharmacology and Biopharmaceutics Review of S-ketamine Tablet. 2019. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000ClinPharmR.pdf. Accessed 13 Mar 2024.
US Food and Drug Administration. Multi-discipline Review of Gilteritinib Table. 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/211349Orig1s000MultidisciplineR.pdf. Accessed 13 Mar 2024.
US Food and Drug Administration. Clinical Pharmacology and Biopharmaceutics Review of Cabozantinib Table. 2016. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208692Orig1s000ClinPharmR.pdf. Accessed 13 Mar 2024
US Food and Drug Administration. Clinical Pharmacology Review of Istradefylline Table. 2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/022075Orig1s000ClinPharmR.pdf. Accessed 13 Mar 2024
Baneyx G, Parrott N, Meille C, Iliadis A, Lavé T. Physiologically based pharmacokinetic modeling of CYP3A4 induction by rifampicin in human: influence of time between substrate and inducer administration. Eur J Pharm Sci. 2014;56:1–15.
US Food and Drug Administration. Multi-discipline Review of Lorlatinib Table. 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210868Orig1s000MultidisciplineR.pdf. Accessed 13 Mar 2024
US Food and Drug Administration. Clinical Review of Istradefylline Tablet. 2019. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/022075Orig1s000MedR.pdf. Accessed 13 Mar 2024
Online Drugbank. Pharmacokinetic information of Cabozantinib. 2022. Available at: https://go.drugbank.com/drugs/DB08875. Accessed 13 Mar 2024
Ford S, Sutton K, Lou Y, Zhang Z, Tenorio A, Trezza C, Patel P, Spreen W. Effect of rifampin on the single-dose pharmacokinetics of oral cabotegravir in healthy subjects. Antimicrob Agents Chemother. 2017;61(10):e00487-e417.
CN Center for Drug Evaluation, NMPA. 药物相互作用研究技术指导原则(试行). 2021. Available at: https://www.cde.org.cn/zdyz/downloadAtt?idCODE=548005195453f85d07c1e9b581e30854. Accessed 13 Mar 2024
Pulte ED, Norsworthy KJ, Wang Y, Xu Q, Qosa H, Gudi R, Przepiorka D, Fu W, Okusanya OO, Goldberg KB. FDA approval summary: gilteritinib for relapsed or refractory acute myeloid leukemia with a FLT3 mutation. Clin Cancer Res. 2021;27(13):3515–21.
Li J, Wu K, Li X, Long S, Zhou Z, Zhao Y, et al. Quantifying Induction/Inhibition Effects on Fuzuloparib Using a Physiologically Based Pharmaco-Kinetic (PBPK) model. J Clin Exp Pathol. 2022;12(4):1000415. Available at: https://www.omicsonline.org/open-access-pdfs/quantifying-inductioninhibition-effects-on-fuzuloparib-using-a-physiologically-based-pharmacokinetic-pbpk-model.pdf
Zhang F, Jia R, Gao H, Wu X, Liu B, Wang H. In silico prediction of bioequivalence of Isosorbide Mononitrate tablets with different dissolution profiles using PBPK modeling and simulation. Eur J Pharm Sci. 2021;157: 105618.
Jia R, Zhang F, Wu N, Xu W, Gao H, Liu B, Wang H. Accelerating Development of Benziamidazole-Class Proton Pump Inhibitors: A Mechanism-Based PK/PD Model to Optimize Study Design with Ilaprazole as a Case Drug. Pharmaceutics. 2021;13(3):392.
Zhang F, Jia R, Gao H, Wu X, Liu B, Wang H. In silico modeling and simulation to guide bioequivalence testing for oral drugs in a virtual population. Clin Pharmacokinet. 2021;60(11):1373–85.
Zhang J, Wu K, B Liu, Hou S, Li X, Ye X, Liu J, He Q. Bioequivalence study of ipratropium bromide inhalation aerosol using PBPK modelling. Frontiers in Medicine. 2023;10:1056318.
Wu X, Zhang F, Yu M, Ding F, Luo J, Liu B, Wang H. Semi-PBPK modeling and simulation to evaluate the local and systemic pharmacokinetics of OC-01 (Varenicline) nasal spray. Front Pharmacol. 2022;13: 910629.
Chao P, Uss AS, Cheng KC. Use of intrinsic clearance for prediction of human hepatic clearance. Expert Opin Drug Metab Toxicol. 2010;6(2):189–98.
Acknowledgements
JL thanks the sponsorship from the Innovation Fund of the Graduate School of Wuhan Institute of Technology (CX2022066). The authors also thank Dr Hongyun Wang at Peking Union Medical College Hospital for providing guidance and scientific suggestions.
Author information
Authors and Affiliations
Contributions
Conceptualization, Bo Liu; Data curation, Jingxi Li, Xiong Jin, Mengjun Zhang; Formal analysis, Keheng Wu Xinyi, Wu, Zhijun Huang; Methodology, Keheng Wu; Project administration, Bo Liu; Software, Keheng Wu & Youni Zhao; Validation, Jingxi Li; Writing-original draft, Jingxi Li; Writing review & editing, Zhou Zhou, Xue Li, Sihui Long and Jack Liu.
Corresponding author
Ethics declarations
Conflict of Interests
Xinyi Wu, Zhijun Huang, Zhou Zhou, Xue Li, Keheng Wu, Youni Zhao, Jack Liu were employees of Yinghan Pharmaceutical Technology (Shanghai) at the time of study conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Li, X., Wu, K. et al. Predicting Drug-Drug Interactions Involving Rifampicin Using a Semi-mechanistic Hepatic Compartmental Model. Pharm Res 41, 699–709 (2024). https://doi.org/10.1007/s11095-024-03691-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-024-03691-5