Abstract
Purpose
New solutions are needed to enable the efficient use of poorly water-soluble drugs. Therefore, we aimed to demonstrate that decreasing particle size with a solution-to-particle method known as nanoforming can improve dissolution and thus bioavailability.
Methods
Piroxicam, a poorly water-soluble non-steroidal anti-inflammatory drug (NSAID), was used as a model compound. A Quality-by-Design (QbD) approach was used to nanoform piroxicam and a design space was established. The pharmacokinetics of piroxicam nanoparticles were compared to two marketed products in a clinical trial.
Results
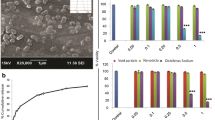
Nanoformed tablets showed a 33% increase in exposure during the first hour after dosing (AUC0–1 h) compared with an immediate release tablet and was similar to a fast absorbing tablet incorporating complexation of piroxicam with β-cyclodextrin.
Conclusions
The results show that nanoforming enabled more rapid absorption in comparison to a typical marketed tablet and indicate that nanoforming is an alternative to complex formulation such as cyclodextrins based products. The study outcomes support the potential of nanoforming for producing fast-acting dosage forms of poorly soluble drugs.




Similar content being viewed by others
Change history
01 January 2024
Article has been updated to remove supplementary information citation on pages 2318 and 2324.
References
Lu Y, Chen Y, Gemeinhart RA, Wu W, Li T. Developing nanocrystals for cancer treatment. Nanomedicine. 2015;10(16):2537–52. https://doi.org/10.2217/nnm.15.73.
Lu Y, Li Y, Wu W. Injected nanocrystals for targeted drug delivery. Acta Pharm Sin B. 2016;6(2):106–13. https://doi.org/10.1016/j.apsb.2015.11.005.
Shohin IE, Kulinich JI, Ramenskaya GV, Abrahamsson B, Kopp S, Langguth P, et al. Biowaiver monographs for immediate release solid oral dosage forms: piroxicam. J Pharm Sci. 2014;103(2):367–77. https://doi.org/10.1002/jps.23799.
Piroxicam. FDA Professional Drug Information. https://www.drugs.com/pro/piroxicam.html. Accessed 25 Jan 2022.
Feldene Capsules. NDA 18-147/S-029 Pfizer. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018147s029lbl.pdf. Accessed 25 Jan 2022.
Woolf AD, Rogers HJ, Bradbrook ID, Corless D. Pharmacokinetic observations on piroxicam in young adult, middle-aged and elderly patients. Brit J Clin Pharmaco. 1983;16(4):433–7. https://doi.org/10.1111/j.1365-2125.1983.tb02191.x.
Piroxicam. Drugbank. https://go.drugbank.com/drugs/DB00554. Accessed 25 Jan 2022.
Perini JA, Vianna-Jorge R, Brogliato AR, Suarez-Kurtz G. Influence of CYP2C9 genotypes on the pharmacokinetics and pharmacodynamics of piroxicam. Clin Pharmacol Ther. 2005;78(4):362–9. https://doi.org/10.1016/j.clpt.2005.06.014.
Piroxicam - Article 31 referral - Annex I, II, III, IV. 2008. EMA. https://www.ema.europa.eu/en/documents/referral/piroxicam-article-31-referral-annex-i-ii-iii-iv_en.pdf. Accessed 25 Jan 2022.
Scarpignato C. Piroxicam-β-cyclodextrin: a GI safer piroxicam. Curr Med Chem. 2013;20(19):2415–37. https://doi.org/10.2174/09298673113209990115.
Pessi J, Lassila I, Meriläinen A, Räikkönen H, Hæggström E, Yliruusi J. Controlled expansion of supercritical solution: a robust method to produce pure drug nanoparticles with narrow size-distribution. J Pharm Sci. 2016;105(8):2293–7. https://doi.org/10.1016/j.xphs.2016.05.022.
Cheeti S, Jou HH, Nelson E, Walker H, Chen B, Morley R, et al. Application of a novel ‘Make and Test in Parallel’ strategy to investigate the effect of formulation on the pharmacokinetics of GDC-0810 in healthy subjects. Pharm Res. 2018;35(12):233. https://doi.org/10.1007/s11095-018-2516-0.
Lobo ED, Argentine MD, Sperry DC, Connor A, McDermott J, Stevens L, et al. Optimization of LY545694 tosylate controlled release tablets through pharmacoscintigraphy. Pharm Res. 2012;29(10):2912–25. https://doi.org/10.1007/s11095-012-0798-1.
McDermott J, Connor A, Sidhu S, Mayes B, Moussa A, Ganga S. Rapid formulation development and clinical evaluation of enabled formulations of IDX–719. In: AAPS annual meeting 2014. San Diego, CA.
Scholes P, Stevens L, Patersom M, Egerton M. Translational Pharmaceutics – interactive drug development to enable rapid optimisation of drug products in early development. In: AAPS annual meeting 2009. Los Angeles, CA.
FDA. Guidance for Industry. Food-Effect Bioavailability and fed bioequivalence studies. 2002. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/food-effect-bioavailability-and-fed-bioequivalence-studies. Accessed 25 Jan 2022.
EMA. Guideline on the Investigation of Bioequivalence. 2010. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf. Accessed 25 Jan 2022.
Dean L. Piroxicam Therapy and CYP2C9 Genotype. Med Gen Sum [Internet], 2019. Feb 11, 2019. Piroxicam Therapy and CYP2C9 Genotype - Medical Genetics Summaries - NCBI Bookshelf (nih.gov). Accessed 25 Jan 2022.
Theken KN, Lee CR, Gong L, Caudle KE, Formea CM, Gaedigk A, et al. Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and nonsteroidal anti-inflammatory drugs. Clin Pharmacol Ther. 2020;108(2):191–200. https://doi.org/10.1002/cpt.1830.
Upadhyay PP, Bond AD. Crystallization and disorder of the polytypic α1 and α2 polymorphs of piroxicam. Cryst Eng Comm. 2015;17(28):5266–72. https://doi.org/10.1039/C5CE00050E.
Sheth AR, Bates S, Muller FX, Grant DJW. Polymorphism in piroxicam. Cryst Growth Des. 2004;4(6):1091–8. https://doi.org/10.1021/cg049876y.
Vrečer F, Vrbinc M, Meden A. Characterization of piroxicam crystal modifications. Int J Pharm. 2003;256(1):3–15. https://doi.org/10.1016/s0378-5173(03)00057-7.
Merah A, Abidi A, Chaffai N, Bataille B, Gherraf N. Role of hydroxypropylmethylcellulose (HPMC 4000) in the protection of the polymorphs of Piroxicam extended release tablets. Arab J Chem. 2017;10(1):S1243–53. https://doi.org/10.1016/j.arabjc.2013.03.005.
Tantishaiyakul VP, Permkam P, Suknuntha K. Use of drifts and PLS for the determination of polymorphs of piroxicam alone and in combination with pharmaceutical excipients: a technical note. AAPS PharmSciTech. 2008;9(1):95–9.
European Pharmacopoeia v. 10, 2.9.3 Dissolution test for solid dosage forms. European Directorate of the Quality of Medicines & Healthcare.
Zecchi V, Orienti I, Fini A. Control of NSAID dissolution by β-cyclodextrin complexation. Pharm Acta Helv. 1988;63:299–302.
Higashino H, Hasegawa T, Yamamoto M, Matsui R, Masaoka Y, Kataoka M, et al. In vitro-in vivo correlation of the effect of supersaturation on the intestinal absorption of BCS Class 2 drugs. Mol Pharm. 2014;11(3):746–54. https://doi.org/10.1021/mp400465p.
Karim A, Noveck R, McMahon FG, Smith M, Crosby S, Adams H, et al. Oxaprozin and piroxicam, nonsteroidal antiinflammatory drugs with long half-lives: effect of protein-binding differences on steady-state pharmacokinetics. J Clin Pharmacol. 1997;37(4):267–78. https://doi.org/10.1002/j.1552-4604.1997.tb04302.x.
Consoli G, Covelli M, Di Matteo L, Marcolongo R, Tirri G, La Montagna G, et al. [Fast-dissolving sublingual tablets of piroxicam versus naproxen in the treatment of recurrent acute osteoarthrosis. Multicenter clinical trial]. Minerva Med. 1994;85(3):89–96.
Desjardins PJ. Analgesic efficacy of piroxicam in postoperative dental pain. Am J Med. 1988;84(5):35–41. https://doi.org/10.1016/0002-9343(88)90475-5.
Richy F, Scarpignato C, Lanas A, Reginster J-Y. Efficacy and safety of piroxicam revisited. A global meta-analysis of randomised clinical trials. Pharmacol Res. 2009;60(4):254–63. https://doi.org/10.1016/j.phrs.2009.03.021.
Skiba M, Boukhris T, Bounoure F, Hatem F. Pharmacokinetic study of an oral piroxicam formulation containing different molar ratios of β-cyclodextrins. J Inc Phenom Macro. 2013;75(3):311–4. https://doi.org/10.1007/s10847-012-0166-0.
Acknowledgements
The authors want to acknowledge all people who contributed to the study at Nanoform, Quotient Sciences, Medfiles, and Particle Analytical.
Funding
No funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors report no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lakio, S., Smith, D.J., Andrade, G. et al. Small is Powerful: Demonstration of the Impact of Nanoformed Piroxicam in a Controlled Clinical Study. Pharm Res 40, 2317–2327 (2023). https://doi.org/10.1007/s11095-023-03624-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03624-8