Abstract
Purpose
For successful delivery of a solid vaccine formulation into the skin using microneedles, the solubility of an adjuvant should be considered because the decrease in the dissolution rate by the addition of adjuvant decreases the delivery efficiency of the vaccine.
Methods
In this study, cholera toxin A subunit 1 (CTA1) was examined as an adjuvant to Hepatitis B vaccine (HBV) microneedles because of its good water solubility, improved safety, and positive effect as shown in intramuscular administration of a liquid vaccine.
Results
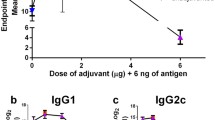
All solid formulations with CTA 1 dissolved in in vivo mouse skin within 30 min, and they were successfully delivered into the skin. In experiments with mice, the addition of CTA1 led to improved IgG immune response compared to the use of an aluminum hydroxide–based formulation and intramuscular administration of HBV. In addition, CTA1 induced CD8 + T cell response as much as in which the aluminum hydroxide–based formulation induced.
Conclusions
CTA1 is an adjuvant that satisfies both the delivery efficiency and the immunological characteristics required for vaccine microneedles. CTA1 will be used as a potential adjuvant through vaccine microneedles.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- Alum:
-
Aluminum hydroxide
- CT:
-
Cholera toxin
- CTA1:
-
Cholera toxin A subunit 1
- HBV microneedle:
-
Hepatitis B vaccine microneedle
- HBsAg:
-
Hepatitis B surface antigen
- HA:
-
Hyaluronic acid
- HBsAg-microneedles:
-
Microneedles coated with a formulation of HBsAg only
- HBsAg-Alum-microneedles:
-
Microneedles coated with a formulation of HBsAg and Alum
- HBsAg-CTA1-microneedles:
-
Microneedles coated with a formulation of HBsAg and CTA1
- HRP:
-
Horseradish peroxidase
- ELISA:
-
Enzyme-linked immunosorbent assay
- ELISpot:
-
Enzyme-linked immunosorbent spot
- OD:
-
Optical density
- SDS-PAGE:
-
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
References
Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M, Dua K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–58.
van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans) dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–55.
Bagley KC, Lewis GK, Fouts TR. Adjuvant activity of the catalytic A1 domain of cholera toxin for retroviral antigens delivered by GeneGun. Clin Vaccine Immunol. 2011;18(6):922–30.
Verbaan F, Bal S, Van den Berg D, Groenink W, Verpoorten H, Lüttge R, Bouwstra J. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J Control Release. 2007;117(2):238–45.
Kim JS, Choi J-A, Kim JC, Park H, Yang E, Park JS, Song M, Park J-H. Microneedles with dual release pattern for improved immunological efficacy of Hepatitis B vaccine. Int J Pharm. 2020;591:119928.
Jeong H-R, Bae J-Y, Park J-H, Baek S-K, Kim G, Park M-S, Park J-H. Preclinical study of influenza bivalent vaccine delivered with a two compartmental microneedle array. J Control Release. 2020;324:280–8.
Song J-M, Kim Y-C, Lipatov AS, Pearton M, Davis CT, Yoo D-G, Park K-M, Chen L-M, Quan F-S, Birchall JC. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B-and T-cell responses in mice. Clin Vaccine Immunol. 2010;17(9):1381–9.
Lee KJ, Jeong SS, Roh DH, Kim DY, Choi H-K, Lee EH. A practical guide to the development of microneedle systems–In clinical trials or on the market. Int J Pharm. 2020;573:118778.
Halder J, Gupta S, Kumari R, Gupta GD, Rai VK. Microneedle array: applications, recent advances, and clinical pertinence in transdermal drug delivery. J Pharm Innov. 2020:1–8.
Ingrole RS, Azizoglu E, Dul M, Birchall JC, Gill HS, Prausnitz MR. Trends of microneedle technology in the scientific literature, patents, clinical trials and internet activity. Biomaterials. 2021;267:120491.
Kang N-W, Kim S, Lee J-Y, Kim K-T, Choi Y, Oh Y, Kim J, Kim D-D, Park J-H. Microneedles for drug delivery: recent advances in materials and geometry for preclinical and clinical studies. Expert Opin Drug Deliv. 2021;18(7):929–47.
Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. 2009;333:369–93.
Al-Zahrani S, Zaric M, McCrudden C, Scott C, Kissenpfennig A, Donnelly RF. Microneedle-mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy. Expert Opin Drug Deliv. 2012;9(5):541–50.
Guillard O, Fauconneau B, Pineau A, Marrauld A, Bellocq J-P, Chenard M-P. Aluminium overload after 5 years in skin biopsy following post-vaccination with subcutaneous pseudolymphoma. J Trace Elem Med Biol. 2012;26(4):291–3.
Bian Q, Xu YH, Ma XL, Hu JY, Gu YT, Wang RX, Yuan AR, Hu WT, Huang LL, Li N. Differential dual-release bilayer microneedles loaded with aluminum adjuvants as a safe and effective vaccine platform. Adv Funct Mater. 2022;32(29):2201952.
Portuondo DL, Batista-Duharte A, Ferreira LS, de Andrade CR, Quinello C, Téllez-Martínez D, de Aguiar Loesch ML, Carlos IZ. Comparative efficacy and toxicity of two vaccine candidates against Sporothrix schenckii using either Montanide™ Pet Gel A or aluminum hydroxide adjuvants in mice. Vaccine. 2017;35(34):4430–6.
Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82(5):488–96.
Butler N, Voyce M, Burland W, Hilton ML. Advantages of aluminium hydroxide adsorbed combined diphtheria, tetanus, and pertussis vaccines for the immunization of infants. Br Med J. 1969;1(5645):663–6.
Chen X, Pravetoni M, Bhayana B, Pentel PR, Wu MX. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine. 2012;31(1):159–64.
Clausi AL, Merkley SA, Carpenter JF, Randolph TW. Inhibition of aggregation of aluminum hydroxide adjuvant during freezing and drying. J Pharm Sci. 2008;97(6):2049–61.
Wolff L, Flemming J, Schmitz R, Gröger K, Müller-Goymann C. Protection of aluminum hydroxide during lyophilisation as an adjuvant for freeze-dried vaccines. Colloids Surf A: Physicochem Eng Asp. 2008;330(2–3):116–26.
Hirschberg H, van Kuijk S, Loch J, Jiskoot W, Bouwstra J, Kersten G, Amorij J-P. A combined approach of vesicle formulations and microneedle arrays for transcutaneous immunization against hepatitis B virus. Eur J Pharm Sci. 2012;46(1–2):1–7.
Oh Y-J, Cha H-R, Hwang SJ, Kim D-S, Choi Y-J, Kim Y-S, Shin Y-R, Nguyen TT, Choi S-O, Lee JM. Ovalbumin and cholera toxin delivery to buccal mucus for immunization using microneedles and comparison of immunological response to transmucosal delivery. Drug Deliv Transl Res. 2021;11(4):1390–400.
Shin J-H, Noh J-Y, Kim K-H, Park J-K, Lee J-H, Jeong SD, Jung D-Y, Song C-S, Kim Y-C. Effect of zymosan and poly (I: C) adjuvants on responses to microneedle immunization coated with whole inactivated influenza vaccine. J Control Release. 2017;265:83–92.
Duong HTT, Yin Y, Thambi T, Nguyen TL, Phan VG, Lee MS, Lee JE, Kim J, Jeong JH, Lee DS. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials. 2018;185:13–24.
Weldon WC, Zarnitsyn VG, Esser ES, Taherbhai MT, Koutsonanos DG, Vassilieva EV, Skountzou I, Prausnitz MR, Compans RW. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS ONE. 2012;7(7):e41501.
Park S, Lee Y, Kwon Y-M, Lee Y-T, Kim K-H, Ko E-J, Jung JH, Song M, Graham B, Prausnitz MR. Vaccination by microneedle patch with inactivated respiratory syncytial virus and monophosphoryl lipid A enhances the protective efficacy and diminishes inflammatory disease after challenge. PLoS ONE. 2018;13(10):e0205071.
Kim E, Erdos G, Huang S, Kenniston TW, Balmert SC, Carey CD, Raj VS, Epperly MW, Klimstra WB, Haagmans BL. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine. 2020;55:102743.
Leone M, Mönkäre J, Bouwstra JA, Kersten G. Dissolving microneedle patches for dermal vaccination. Pharm Res. 2017;34(11):2223–40.
Donnelly RF, Garland MJ, Morrow DI, Migalska K, Singh TRR, Majithiya R, Woolfson AD. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J Control Release. 2010;147(3):333–41.
Zhao X, Coulman SA, Hanna SJ, Wong FS, Dayan CM, Birchall JC. Formulation of hydrophobic peptides for skin delivery via coated microneedles. J Control Release. 2017;265:2–13.
Paredes AJ, McKenna PE, Ramöller IK, Naser YA, Volpe-Zanutto F, Li M, Abbate M, Zhao L, Zhang C, Abu-Ershaid JM. Microarray patches: poking a hole in the challenges faced when delivering poorly soluble drugs. Adv Funct Mater. 2021;31(1):2005792.
Ono A, Ito S, Sakagami S, Asada H, Saito M, Quan Y-S, Kamiyama F, Hirobe S, Okada N. Development of novel faster-dissolving microneedle patches for transcutaneous vaccine delivery. Pharmaceutics. 2017;9(3):27.
Arya J, Henry S, Kalluri H, McAllister DV, Pewin WP, Prausnitz MR. Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials. 2017;128:1–7.
Jeong H-R, Lee H-S, Choi I-J, Park J-H. Considerations in the use of microneedles: pain, convenience, anxiety and safety. J Drug Target. 2017;25(1):29–40.
Bharati K, Ganguly NK. Cholera toxin: a paradigm of a multifunctional protein. Indian J Med Res. 2011;133(2):179.
Lycke N. The mechanism of cholera toxin adjuvanticity. Res Immunol. 1997;148(8):504–20.
Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol Today. 1999;20(11):493–500.
Kenneth C, Bagley GKL, Timothy R. Fouts. Adjuvant activity of the catalytic A1 domain of cholera toxin for retroviral antigens delivered by genegun. Clin Vaccine Immunol. 2011;18:922–930.
Ding Z, Van Riet E, Romeijn S, Kersten G, Jiskoot W, Bouwstra J. Immune modulation by adjuvants combined with diphtheria toxoid administered topically in BALB/c mice after microneedle array pretreatment. Pharm Res. 2009;26(7):1635–43.
Menon I, Bagwe P, Gomes KB, Bajaj L, Gala R, Uddin MN, D’souza MJ, Zughaier SM. Microneedles: a new generation vaccine delivery system. Micromachines. 2021;12(4):435.
Kim S-H, Jang Y-S. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin Exp Vaccine Res. 2017;6(1):15–21.
Eliasson DG, El Bakkouri K, Schön K, Ramne A, Festjens E, Löwenadler B, Fiers W, Saelens X, Lycke N. CTA1-M2e-DD: a novel mucosal adjuvant targeted influenza vaccine. Vaccine. 2008;26(9):1243–52.
Lycke N, Bemark M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins. Mucosal Immunol. 2010;3(6):556–66.
Shim B-S, Cheon IS, Lee E, Park S-M, Choi Y, Jung D-I, Yang E, Choi J-A, Chun JY, Kim J-O. Development of safe and non-self-immunogenic mucosal adjuvant by recombinant fusion of cholera toxin A1 subunit with protein transduction domain. J Immunol Res. 2018;2018:1–11.
Su F, Patel GB, Hu S, Chen W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum Vaccines Immunother. 2016;12(4):1070–9.
Nguyen TT, Choi J-A, Kim JS, Park H, Yang E, Lee WJ, Baek S-K, Song M, Park J-H. Skin immunization with third-generation hepatitis B surface antigen using microneedles. Vaccine. 2019;37(40):5954–61.
Kool M, Fierens K, Lambrecht BN. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol. 2012;61(7):927–34.
Snapper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236(4804):944–7.
Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334(6179):255–8.
Li X, Yang X, Jiang Y, Liu J. A novel HBV DNA vaccine based on T cell epitopes and its potential therapeutic effect in HBV transgenic mice. Int Immunol. 2005;17(10):1293–302.
Funding
This work was supported by National Research Foundation of Korea (NRF) grants funded by Korea Ministry of Trade, Industry & Energy (MOTIE, 20014911 (Industrial Strategic Technology Development Program)), by Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HV22C0029).
Author information
Authors and Affiliations
Contributions
Conceptualization: P.J., H.H.; Methodology & Investigation: K.G, S.G., P.J., J.W.; Software: M.J and G.W, K.J; Formal Analysis: B.S.; Writing & Draft Preparation: K.J., P.J., H.H.; Writing-review& Editing: all authors; Supervision: P.J., H.H.; Funding Acquisition: B.S., H.H. Data curation: H.H., P.J., P.J.
Corresponding authors
Ethics declarations
Conflict of Interest
PJH is an inventor of patents that have been licensed to companies developing microneedle-based products, is a shareholder of companies developing microneedle-based products.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, JC., Choi, Ja., Park, H. et al. Pharmaceutical and Immunological Evaluation of Cholera Toxin A1 Subunit as an Adjuvant of Hepatitis B Vaccine Microneedles. Pharm Res 40, 3059–3071 (2023). https://doi.org/10.1007/s11095-023-03623-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03623-9