Abstract
Background
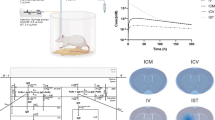
Dosing regimens of trastuzumab administered by intracerebroventricular (icv) route to patients with HER2-positive brain localizations remain empirical. The objectives of this study were to describe pharmacokinetics (PK) of trastuzumab in human plasma and cerebrospinal fluid (CSF) after simultaneous icv and intravenous (iv) administration using a minimal physiologically-based pharmacokinetic model (mPBPK) and to perform simulations of alternative dosing regimens to achieve therapeutic concentrations in CSF.
Methods
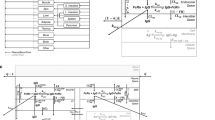
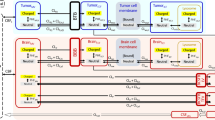
Plasma and CSF PK data were collected in two patients with HER2-positive brain localizations. A mPBPK model for mAbs consisting of four compartments (tight and leaky tissues, plasma and lymph) was enriched by an additional compartment for ventricular CSF. The comparison between observed and model-predicted concentrations was evaluated using prediction error (PE).
Results
The developed mPBPK model described plasma and CSF trastuzumab concentrations reasonably well with mean PE for plasma and CSF data of 41.8% [interquartile range, IQR = -9.48; 40.6] and 18.3% [-36.7; 60.6], respectively, for patient 1 and 11.4% [-10.8; 28.7] and 22.5% [-27.7; 77.9], respectively, for patient 2. Trastuzumab showed fast clearance from CSF to plasma with Cmin,ss of 0.56 and 0.85 mg/L for 100 and 150 mg q1wk, respectively. Repeated dosing of 100 and 150 mg q3day resulted in Cmin,ss of 10.3 and 15.4 mg/L, respectively. Trastuzumab CSF target concentrations are achieved rapidly and maintained above 60 mg/L from 7 days after a continuous perfusion at 1.0 mg/h.
Conclusion
Continuous icv infusion of trastuzumab at 1.0 mg/h could be an alternative dosing regimen to rapidly achieve intraventricular CSF therapeutic concentrations.



Similar content being viewed by others
Data Availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Abouharb S, Ensor J, Loghin ME, Katz R, Moulder SL, Esteva FJ, et al. Leptomeningeal disease and breast cancer: The importance of tumor subtype. Breast Cancer Res Treat. 2014;146:477–86. Available from: https://link.springer.com/article/10.1007/s10549-014-3054-z.
Lazaratos AM, Maritan SM, Quaiattini A, Darlix A, Ratosa I, Ferraro E, et al. Intrathecal trastuzumab versus alternate routes of delivery for HER2-targeted therapies in patients with HER2+ breast cancer leptomeningeal metastases. Breast. 2023;0. Available from: http://www.thebreastonline.com/article/S0960977623004502/fulltext.
Tan YO, Han S, Lu YS, Yip CH, Sunpaweravong P, Jeong J, et al. The prevalence and assessment of ErbB2-positive breast cancer in Asia. Cancer. 2010;116:5348–57. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/cncr.25476.
Thomas KH, Ramirez RA. Leptomeningeal disease and the evolving role of molecular targeted therapy and immunotherapy. Ochsner J. 2017;17:362.
Stemmler H-J, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18:23–8. Available from: https://insights.ovid.com/crossref?an=00001813-200701000-00004.
Stemmler HJ, Schmitt M, Harbeck N, Willems A, Bernhard H, Lässig D, et al. Application of intrathecal trastuzumab (HerceptinTM) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncol Rep. 2006;15:1373–7. Available from: http://www.spandidospublications.com/10.3892/or.15.5.1373/abstract.
Le Tilly O, Azzopardi N, Bonneau C, Desvignes C, Oberkampf F, Ezzalfani M, et al. Antigen mass may influence trastuzumab concentrations in cerebrospinal fluid after intrathecal administration. Clin Pharmacol Ther. 2021;110:210–9. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/cpt.2188.
Baselga J. Phase I and II clinical trials of trastuzumab. Ann Oncol. 2001;12:S49–55. Available from: http://www.annalsofoncology.org/article/S0923753419544581/fulltext.
Bonneau C, Paintaud G, Trédan O, Dubot C, Desvignes C, Dieras V, et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018;95:75–84. Available from: http://www.ejcancer.com/article/S0959804918307147/fulltext.
Kadoch C, Li J, Wong VS, Chen L, Cha S, Munster P, et al. Complement activation and intraventricular rituximab distribution in recurrent central nervous system lymphoma. Clin Cancer Res. Am Assoc Cancer Res. 2014;20:1029–41. Available from: https://aacrjournals.org/clincancerres/article/20/4/1029/78802/Complement-Activation-and-Intraventricular.
Bousquet G, Darrouzain F, De Bazelaire C, Ternant D, Barranger E, Winterman S, et al. Intrathecal trastuzumab halts progression of CNS metastases in breast cancer. J Clin Oncol. Am Soc Clin Oncol. 2016;34:e151–5.
Chang HY, Wu S, Meno-Tetang G, Shah DK. A translational platform PBPK model for antibody disposition in the brain. J Pharmacokinet Pharmacodyn. 2019;46:319–38. Available from: https://link.springer.com/article/10.1007/s10928-019-09641-8.
Bloomingdale P, Bakshi S, Maass C, van Maanen E, Pichardo-Almarza C, Yadav DB, et al. Minimal brain PBPK model to support the preclinical and clinical development of antibody therapeutics for CNS diseases. J Pharmacokinet Pharmacodyn. 2021;48:861–71. Available from: https://link.springer.com/article/10.1007/s10928-021-09776-7.
Willeman T, Jourdil JF, Gautier-Veyret E, Bonaz B, Stanke-Labesque F. A multiplex liquid chromatography tandem mass spectrometry method for the quantification of seven therapeutic monoclonal antibodies: Application for adalimumab therapeutic drug monitoring in patients with Crohn’s disease. Anal Chim Acta Elsevier. 2019;1067:63–70.
Cao Y, Balthasar JP, Jusko WJ. Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn. 2013;40:597–607. Available from: https://link.springer.com/article/10.1007/s10928-013-9332-2.
Sarin H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res. 2010;2:1–19. Available from: https://vascularcell.biomedcentral.com/articles/10.1186/2040-2384-2-14.
Stücker O, Pons-Himbert EL C. Towards a better understanding of lymph circulation. Phlebolymphology. 2008;15(1):31.
Keizer RJ, Jansen RS, Rosing H, Thijssen B, Beijnen JH, Schellens JHM, et al. Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect. 2015;3:1–15.
Sutter P-J De, Cock P De, Johnson TN, Musther H, Gasthuys E, Vermeulen A. Predictive performance of physiologically based pharmacokinetic modelling of beta-lactam antibiotic concentrations in adipose, bone and muscle tissues. Drug Metab Dispos. 2023;DMD-AR-2022–001129. Available from: https://dmd.aspetjournals.org/content/early/2023/01/13/dmd.122.001129.
Quartino AL, Li H, Kirschbrown WP, Mangat R, Wada DR, Garg A, et al. Population pharmacokinetic and covariate analyses of intravenous trastuzumab (Herceptin ® ), a HER2-targeted monoclonal antibody, in patients with a variety of solid tumors. Cancer Chemother Pharmacol. 2019;83:329–40. Available from: https://link.springer.com/article/10.1007/s00280-018-3728-z.
Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn. 2008;35:573–91. Available from: https://link.springer.com/article/10.1007/s10928-008-9102-8.
Li L, Gardner I, Rose R, Jamei M. Incorporating target shedding into a minimal PBPK–TMDD model for monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol. 2014;3:1–13. Available from: https://onlinelibrary.wiley.com/doi/full/10.1038/psp.2013.73.
Petitcollin A, Azzopardi N, Pierga JY, Ternant D, Navarro-Teulon I, Desvignes C, et al. Population pharmacokinetics and exposure–response relationship of trastuzumab and bevacizumab in early-stage breast cancer. Eur J Clin Pharmacol. 2021;77:1861–73. Available from: https://link.springer.com/article/10.1007/s00228-021-03179-w.
Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. John Wiley & Sons, Ltd; 2017;6:58–66.
Marchand M, Zhang R, Chan P, Quarmby V, Ballinger M, Sternheim N, et al. Time-dependent population PK models of single-agent atezolizumab in patients with cancer. Cancer Chemother Pharmacol. 2021;88:211–21. Available from: https://link.springer.com/article/10.1007/s00280-021-04276-4.
Li H, Yu J, Liu C, Liu J, Subramaniam S, Zhao H, et al. Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J Pharmacokinet Pharmacodyn. 2017;44:403–14. Available from: https://link.springer.com/article/10.1007/s10928-017-9528-y.
Abe K, Shibata K, Naito T, Otsuka A, Karayama M, Maekawa M, et al. Impacts of cachexia progression in addition to serum IgG and blood lymphocytes on serum nivolumab in advanced cancer patients. Eur J Clin Pharmacol. 2022;78:77–87. Available from: https://link.springer.com/article/10.1007/s00228-021-03199-6.
Liu C, Yu J, Li H, Liu J, Xu Y, Song P, et al. Association of Time-Varying Clearance of Nivolumab With Disease Dynamics and Its Implications on Exposure Response Analysis. Clin Pharmacol Ther. 2017;101:657–66. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/cpt.656.
Liao S, Von Der Weid PY. Inflammation-induced lymphangiogenesis and lymphatic dysfunction. Angiogenesis. 2014;17:325
Bruno R, Washington CB, Lu JF, Lieberman G, Banken L, Klein P. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol. 2005;56:361–9. Available from: https://link.springer.com/article/10.1007/s00280-005-1026-z.
Zagouri F, Sergentanis TN, Bartsch R, Berghoff AS, Chrysikos D, De Azambuja E, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: A systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139:13–22. Available from: https://link.springer.com/article/10.1007/s10549-013-2525-y.
Kumthekar PU, Avram MJ, Lassman AB, Lin NU, Lee E, Grimm SA, et al. A phase I/II study of intrathecal trastuzumab in human epidermal growth factor receptor 2-positive (HER2-positive) cancer with leptomeningeal metastases: Safety, efficacy, and cerebrospinal fluid pharmacokinetics. Neuro Oncol. 2023;25:557–65. Available from: https://academic.oup.com/neuro-oncology/article/25/3/557/6660706.
Oberkampf F, Gutierrez M, Trabelsi Grati O, Rhun É Le, Trédan O, Turbiez I, et al. Phase II study of intrathecal administration of trastuzumab in patients with HER2-positive breast cancer with leptomeningeal metastasis. Neuro Oncol. 2023;25:365–74. Available from: https://academic.oup.com/neuro-oncology/article/25/2/365/6648801.
Cosson VF, Ng VW, Lehle M, Lum BL. Population pharmacokinetics and exposure-response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Cancer Chemother Pharmacol. 2014;73:737–47. Available from: https://link.springer.com/article/10.1007/s00280-014-2400-5.
Leyland-Jones B, Colomer R, Trudeau ME, Wardley A, Latreille J, Cameron D, et al. Intensive loading dose of trastuzumab achieves higher-than-steady-state serum concentrations and is well tolerated. J Clin Oncol. 2010;28:960–6.
Gonullu B, Angeli E, Pamoukdjian F, Bousquet G. HER2 Amplification level predicts pathological complete response in the neoadjuvant setting of HER2-Overexpressing breast cancer: A Meta-Analysis and Systematic Review. Int J Mol Sci. 2023;24:3590. Available from: https://www.mdpi.com/1422-0067/24/4/3590/htm.
Otani R, Yamada R, Kushihara Y, Inazuka M, Shinoura N. Continuous intrathecal injection therapy of methotrexate is a therapeutic option in primary CNS lymphoma. J Clin Neurosci. 2019;69:26–30. Available from: http://www.jocn-journal.com/article/S0967586819302905/fulltext.
Ordia JI, Fischer E, Adamski E, Chagnon KG, Spatz EL. Continuous intrathecal baclofen infusion by a programmable pump in 131 consecutive patients with severe spasticity of spinal origin. Neuromodulation Technol Neural Interface Elsevier. 2002;5:16–24.
Paul M, Vieillard V, Da Silva LR, Escalup L, Astier A. Long-term physico-chemical stability of diluted trastuzumab. Int J Pharm Elsevier. 2013;448:101–4.
Zhang Y, Pardridge WM. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol. 2001;114:168–72. Available from: http://www.jni-journal.com/article/S0165572801002429/fulltext.
Cooper PR, Ciambrone GJ, Kliwinski CM, Maze E, Johnson L, Li Q, et al. Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor. FcRn Brain Res Elsevier. 2013;1534:13–21.
Garg A, Balthasar JP. Investigation of the influence of FcRn on the distribution of IgG to the brain. AAPS J. 2009;11:553–7. Available from: https://link.springer.com/article/10.1208/s12248-009-9129-9.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–25. Available from: https://www.nature.com/articles/nri2155.
Hirasawa M, de Lange ECM. Revisiting cerebrospinal fluid flow direction and rate in physiologically based pharmacokinetic model. Pharmaceutics. 2022;14:1764. Available from: https://www.mdpi.com/1999-4923/14/9/1764/htm.
Glitza Oliva IC, Ferguson SD, Bassett R, Foster AP, John I, Hennegan TD, et al. Concurrent intrathecal and intravenous nivolumab in leptomeningeal disease: phase 1 trial interim results. Nat Med. 2023;29:898–905. Available from: https://www.nature.com/articles/s41591-022-02170-x.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Puszkiel, A., Bousquet, G., Stanke-Labesque, F. et al. A Minimal PBPK Model for Plasma and Cerebrospinal Fluid Pharmacokinetics of Trastuzumab after Intracerebroventricular Administration in Patients with HER2-Positive Brain Metastatic Localizations. Pharm Res 40, 2687–2697 (2023). https://doi.org/10.1007/s11095-023-03614-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03614-w