Abstract
Purpose
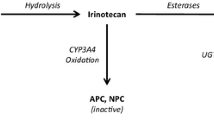
Our previous screening studies identified Oroxylin A (OXA) as a strong inhibitor on the carboxyolesterase mediated hydrolysis of irinotecan to SN-38. The current study employed a whole-body physiologically based pharmacokinetic (PBPK) modeling approach to investigate the underlying mechanisms of the carboxylesterase-mediated pharmacokinetics interactions between irinotecan and OXA in rats.
Methods
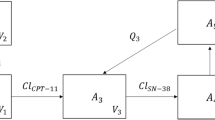
Firstly, rats received irinotecan intravenous treatment at 35 μmol/kg without or with oral OXA pretreatment (2800 μmol/kg) daily for 5 days. On day 5, blood and tissues were collected for analyses of irinotecan/SN-38 concentrations and carboxylesterase expression. In addition, effects of OXA on the enzyme kinetics of irinotecan hydrolysis and unbound fractions of irinotecan and SN-38 in rat plasma, liver and intestine were also determined. Finally, a PBPK model that integrated the physiological parameters, enzyme kinetics, and physicochemical properties of irinotecan and OXA was developed.
Results
Our PBPK model could accurately predict the pharmacokinetic profiles of irinotecan/SN-38, with AUC0-6h and Cmax values within ±27% of observed values. When OXA was included as a carboxylesterase inhibitor, the model could also predict the irinotecan/SN-38 plasma concentrations within twofold of those observed. In addition, the PBPK model indicated inhibition of carboxylesterase-mediated hydrolysis of irinotecan in the intestinal mucosa as the major underlying mechanism for the pharmacokinetics interactions between irinotecan and OXA.
Conclusion
A whole-body PBPK model was successfully developed to not only predict the impact of oral OXA pretreatment on the pharmacokinetics profiles of irinotecan but also reveal its inhibition on the intestinal carboxylesterase as the major underlying mechanism.







Similar content being viewed by others
Abbreviations
- AUC :
-
area under curve
- CAR :
-
constitutive androstane receptor
- CES :
-
Carboxylesterase
- Cmax :
-
peak plasma concentration
- hCE1 :
-
human CES1
- hCE2 :
-
human CES2
- HDI :
-
herb-drug interaction
- IS :
-
internal standard
- IV :
-
intravenous
- K i :
-
inhibition constants
- K m :
-
Michaelis constant
- OAG :
-
oroxylin A-7-O-glucuronide glucuronide
- OXA :
-
oroxylin A
- PO :
-
oral
- PBPK :
-
physiologically based pharmacokinetic
- PXR :
-
pregnane x receptor
- PK :
-
pharmacokinetics
- RS :
-
Radix Scutellariae
- V max :
-
Maximum velocity
References
Fraunfelder FW. Ocular side effects associated with dietary supplements and herbal medicines. Drugs Today (Barc). 2005;41(8):537–45.
Chan WJ, Adiwidjaja J, McLachlan AJ, Boddy AV, Harnett JE. Interactions between natural products and cancer treatments: underlying mechanisms and clinical importance. Cancer Chemother Pharmacol. 2023;91(2):103–19.
Di L. The impact of carboxylesterases in drug metabolism and pharmacokinetics. Curr Drug Metab. 2019;20(2):91–102.
Markovic M, Ben-Shabat S, Dahan A. Computational simulations to guide enzyme-mediated prodrug activation. Int J Mol Sci. 2020;21(10):3621.
Gilbert DC, Chalmers AJ, El-Khamisy SF. Topoisomerase I inhibition in colorectal cancer: biomarkers and therapeutic targets. Br J Cancer. 2012;106(1):18–24.
Parvez MM, Basit A, Jariwala PB, Gaborik Z, Kis E, Heyward S, Redinbo MR, Prasad B. Quantitative investigation of irinotecan metabolism, transport, and gut microbiome activation. Drug Metab Dispos. 2021;49(8):683–93.
Rivory LP, Robert J. Molecular, cellular, and clinical aspects of the pharmacology of 20(S)camptothecin and its derivatives. Pharmacol Ther. 1995;68(2):269–96.
Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, Yokoyama A, Saitoh S, Shimokata K, Hasegawa Y. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res. 2000;60(24):6921–6.
Bhatt AP, Pellock SJ, Biernat KA, Walton WG, Wallace BD, Creekmore BC, Letertre MM, Swann JR, Wilson ID, Roques JR, Darr DB, Bailey ST, Montgomery SA, Roach JM, Azcarate-Peril MA, Sartor RB, Gharaibeh RZ, Bultman SJ, Redinbo MR. Targeted inhibition of gut bacterial beta-glucuronidase activity enhances anticancer drug efficacy. Proc Natl Acad Sci U S A. 2020;117(13):7374–81.
Yang AK, He SM, Liu L, Liu JP, Wei MQ, Zhou SF. Herbal interactions with anticancer drugs: mechanistic and clinical considerations. Curr Med Chem. 2010;17(16):1635–78.
Zhang J, Xiao M, Ji X, Lai YS, Song Q, Zhang Y, Ip CM, Ng WL, Zuo Z. Inhibition of Radix Scutellariae flavones on carboxylesterase mediated activations of prodrugs. Life Sci. 2022;305:120743.
Ren G, Chen H, Zhang M, Yang N, Yang H, Xu C, Li J, Ning C, Song Z, Zhou S, Bian Y, Lu Y, Li N, Zhang Y, Chen X, Zhao D. Pharmacokinetics, tissue distribution and excretion study of Oroxylin a, Oroxylin a 7-O-glucuronide and Oroxylin a sodium sulfonate in rats after administration of Oroxylin a. Fitoterapia. 2020;142:104480.
Watanabe R, Esaki T, Kawashima H, Natsume-Kitatani Y, Nagao C, Ohashi R, Mizuguchi K. Predicting fraction unbound in human plasma from chemical structure: improved accuracy in the low value ranges. Mol Pharm. 2018;15(11):5302–11.
Shelby MK, Cherrington NJ, Vansell NR, Klaassen CD. Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metab Dispos. 2003;31(3):326–33.
Mai Y, Dou L, Yao Z, Madla CM, Gavins FKH, Taherali F, Yin H, Orlu M, Murdan S, Basit AW. Quantification of P-glycoprotein in the gastrointestinal tract of humans and rodents: methodology, gut region, sex, and species matter. Mol Pharm. 2021;18(5):1895–904.
Zhang LL, Lu L, Jin S, Jing XY, Yao D, Hu N, Liu L, Duan R, Liu XD, Wang GJ, Xie L. Tissue-specific alterations in expression and function of P-glycoprotein in streptozotocin-induced diabetic rats. Acta Pharmacol Sin. 2011;32(7):956–66.
Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326(1):181–7.
Meyer M, Schneckener S, Ludewig B, Kuepfer L, Lippert J. Using expression data for quantification of active processes in physiologically based pharmacokinetic modeling. Drug Metab Dispos. 2012;40(5):892–901.
Jin Q, Feng L, Wang DD, Dai ZR, Wang P, Zou LW, Liu ZH, Wang JY, Yu Y, Ge GB, Cui JN, Yang L. A two-photon Ratiometric fluorescent probe for imaging carboxylesterase 2 in living cells and tissues. ACS Appl Mater Interfaces. 2015;7(51):28474–81.
Staudinger JL, Xu C, Cui YJ, Klaassen CD. Nuclear receptor-mediated regulation of carboxylesterase expression and activity. Expert Opin Drug Metab Toxicol. 2010;6(3):261–71.
Xu C, Wang X, Staudinger JL. Regulation of tissue-specific carboxylesterase expression by pregnane x receptor and constitutive androstane receptor. Drug Metab Dispos. 2009;37(7):1539–47.
Acknowledgments
This work was supported by the General Research Fund of Hong Kong Research Grants Council (Project number: 14108119). The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 208 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Zhang, Y., Lai, Y.S. et al. Elucidation of Carboxylesterase Mediated Pharmacokinetic Interactions between Irinotecan and Oroxylin A in Rats via Physiologically Based Pharmacokinetic Modeling. Pharm Res 40, 2627–2638 (2023). https://doi.org/10.1007/s11095-023-03590-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03590-1