Abstract
Purpose
In vitro release testing (IVRT) is a widely used tool for evaluating the quality and performance of drug products. However, standardized sample adaptors or drug release apparatus setups for IVRT studies are still lacking for ophthalmic ointments. The aim of this study was to provide a better understanding of the impact of apparatus and sample adaptor setups on IVRT of ophthalmic ointments.
Methods
Dexamethasone (DEX), a steroidal ingredient commonly used in ophthalmic drug products, was selected as a model drug. Ointments were prepared by mixing DEX in white petrolatum using a high shear mixer. A novel two-sided adapter was developed to increase the drug release surface area. DEX ointment was placed in one-sided or two-sided release adaptors coupled with 1.2 μm polyethersulfone membrane, and the drug release was studied in different USP apparatuses (I, II, and IV).
Results
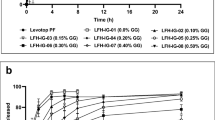
The sample adaptor setups had a minimal impact on cumulative drug release amount per area or release rate while USP IV apparatus with agitated flow enhanced drug release rates. The USP apparatus I with a two-sided semisolid adapter, which uses membranes on both sides, showed dramatically higher cumulative drug release and discriminative release profiles when evaluating ophthalmic formulations.
Conclusions
USP apparatuses and sample adaptors are critical considerations for IVRT. Two-sided semisolid adapter provides higher cumulative release, facilitating the discrimination between low drug content ophthalmic ointment formulations with good sensitivity and repeatability without affecting the drug release rate.










Similar content being viewed by others
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
References
Xu X, et al. Kinetics of drug release from ointments: role of transient-boundary layer. Int J Pharm. 2015;494(1):31–9.
SUPAC-SS: Nonsterile Semisolid Dosage Forms; Scale-Up and Post-Approval Changes: Chemistry, Manufacturing and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation. In: FDA Guidance Documents for Industry. 1997. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-ss-nonsterile-semisolid-dosage-forms-scale-and-post-approval-changes-chemistrymanufacturing. Accessed 15 Aug 2023.
Sripetch S, Loftsson T. Topical drug delivery to the posterior segment of the eye: thermodynamic considerations. Int J Pharm. 2021;597:120332.
Mazet R, et al. Recent advances in the design of topical ophthalmic delivery systems in the treatment of ocular surface inflammation and their biopharmaceutical evaluation. Pharmaceutics. 2020;12(6):570.
Hardberger R, Hanna C, Boyd CM. Effects of drug vehicles on ocular contact time. Arch Ophthalmol. 1975;93(1):42–5.
Bao Q, Burgess DJ. Perspectives on physicochemical and in vitro profiling of ophthalmic ointments. Pharm Res. 2018;35:1–12.
Choi SH, Lionberger RA. Clinical, pharmacokinetic, and in vitro studies to support bioequivalence of ophthalmic drug products. AAPS J. 2016;18:1032–8.
Zhang X, et al. Innovative approaches for demonstration of bioequivalence: the US FDA perspective. Ther Deliv. 2013;4(6):725–40.
Dong Y, et al. Formulation characteristics and in vitro release testing of cyclosporine ophthalmic ointments. Int J Pharm. 2018;544(1):254–64.
Bao Q, et al. Physicochemical attributes and dissolution testing of ophthalmic ointments. Int J Pharm. 2017;523(1):310–9.
Al-Ghabeish M, et al. Influence of drug loading and type of ointment base on the in vitro performance of acyclovir ophthalmic ointment. Int J Pharm. 2015;495(2):783–91.
Xu X, et al. Formulation and process factors influencing product quality and in vitro performance of ophthalmic ointments. Int J Pharm. 2015;493(1–2):412–25.
Bao Q, et al. In vitro release testing method development for ophthalmic ointments. Int J Pharm. 2017;526(1–2):145–56.
Bao Q, et al. In vitro and ex vivo correlation of drug release from ophthalmic ointments. J Control Release. 2018;276:93–101.
Marques MR, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011;18(3):15–28.
Patere S, et al. Influence of manufacturing process variables on the properties of ophthalmic ointments of tobramycin. Pharm Res. 2018;35:1–16.
Mekjaruskul C, et al. Impact of membranes on in vitro release assessment: a case study using dexamethasone. AAPS PharmSciTech. 2021;22:1–9.
Kulkarni M, et al. In vitro release testing of acyclovir topical formulations using immersion cells. Assay Drug Dev Technol. 2021;19(2):75–84.
Sirasitthichoke C, et al. Experimental determination of the velocity distribution in USP apparatus 1 (basket apparatus) using particle image velocimetry (PIV). Int J Pharm X. 2021;3:100078.
Okazaki A, Mano T, Sugano K. Theoretical dissolution model of poly-disperse drug particles in biorelevant media. J Pharm Sci. 2008;97(5):1843–52.
Mauro KC. Chapter 3 – Fick’s Laws of Diffusion. In: Mauro KC. Materials Kinetics Transport and Rate Phenomena. Elsevier; 2021, pp. 39–58. https://doi.org/10.1016/B978-0-12-823907-0.00026-1
Patere S, et al. Influence of in vitro release methods on assessment of tobramycin ophthalmic ointments. Int J Pharm. 2020;590:119938.
Funding
Funding for this project was made possible by the Food and Drug Administration through grant 1U01FD005174–01 and contract HHSF223201810114C. The views expressed in this paper do not reflect the official policies of the U.S. Food and Drug Administration or the U.S. Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
The authors of this journal paper disclose potential conflicts of interest related to the research and findings presented. The invention (two-sided semisolid adapter) described in this paper is licensed to SOTAX Corporation (2400 Computer Drive, Westborough, MA 01581 USA). The author, Dr. Xiuling Lu, may receive royalties or financial benefits from the sale or commercialization of this invention through the licensing agreement with SOTAX Corporation. It is important to note that the study design, data collection, analysis, and interpretation of the results were conducted independently and objectively, without any influence from the potential financial gains. The authors affirm that the conclusions drawn and the information provided in this paper are based on rigorous research and scientific evidence.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mekjaruskul, C., O’Reilly Beringhs, A., Qin, B. et al. Impact of Apparatus and Adapter on In vitro Drug Release of Ophthalmic Semisolid Drug Products. Pharm Res 40, 2239–2251 (2023). https://doi.org/10.1007/s11095-023-03586-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03586-x