Abstract
Purpose
5-fluorouracil (5-FU) and its prodrug capecitabine are commonly prescribed anti-tumor medications. We aimed to establish physiologically based pharmacokinetic (PBPK) models of capecitabine-metabolites and 5-FU-metabolites to describe their pharmacokinetics in tumor and plasma of cancer patients with liver impairment.
Methods
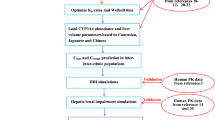
Models including the cancer compartment were developed in PK-Sim® and MoBi® and evaluated by R programming language with 25 oral capecitabine and 18 intravenous 5-FU studies for cancer patients with and without liver impairment.
Results
The PBPK models were constructed successfully as most simulated Cmax and AUClast were within two-fold error of observed values. The simulated alterations of tumor 5-FU Cmax and AUClast in cancer patients with severe liver injury compared with normal liver function were 1.956 and 3.676 after oral administration of capecitabine, but no significant alteration was observed after intravenous injection of 5-FU. Besides, 5-FU concentration in tumor tissue increases with higher tumor blood flow but not tumor size. Sensitivity analysis revealed that dihydropyrimidine dehydrogenase (DPD) and other metabolic enzymes′ activity, capecitabine intestinal permeability and plasma protein scale factor played a vital role in tumor and plasma 5-FU pharmacokinetics.
Conclusions
PBPK model prediction suggests no dosage adaption of capecitabine or 5-FU is required for cancer patients with hepatic impairment but it would be reduced when the toxic reaction is observed. Furthermore, tumor blood flow rate rather than tumor size is critical for 5-FU concentration in tumor. In summary, these models could predict pharmacokinetics of 5-FU in tumor in cancer patients with varying characteristics in different scenarios.









Similar content being viewed by others
Data Availability
Data available on request from the authors, Yu Wang (Email:21919028@zju.edu.cn) or Su Zeng (Email:zengsu@zju.edu.cn).
Abbreviations
- 5′-DFCR:
-
5′-deoxy-5-fluorocytidine
- 5′-DFUR:
-
5′-deoxy-5-fluorouridine
- 5-FU:
-
5-fluorouracil
- FUH2 :
-
Fluorodihydrouracil
- FUPA:
-
α-fluoro-β-ureidopropionic acid
- FBAL:
-
α-fluoro-β-alanine
- CES:
-
Carboxylesterase
- CDA:
-
Cytidine deaminase
- TP:
-
Thymidine phosphorylase
- DPD:
-
Dihydropyrimidine dehydrogenase
- DHP:
-
Dihydropyrimidase
- BUP:
-
β-ureidopropionase
- OPRT:
-
Orotate phosphoribosyltransferase
- CP-B:
-
Child-Pugh B
- CP-C:
-
Child-Pugh C
- GOF:
-
Goodness-of-fit
- GMFE:
-
Geometric mean fold-error
- AUClast :
-
Area under the plasma concentration–time curve from time zero to the time of the last quantifiable concentration
References
Reigner B, Blesch K, Weidekamm E. Clinical pharmacokinetics of capecitabine. Clin Pharmacokinet. 2001;40(2):85–104.
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer. 1998;34(8):1274–81.
Thorn CF, Marsh S, Carrillo MW, McLeod HL, Klein TE, Altman RB. PharmGKB summary: fluoropyrimidine pathways. Pharmacogenet Genomics. 2011;21(4):237–42.
Derissen EJ, Jacobs BA, Huitema AD, Rosing H, Schellens JH, Beijnen JH. Exploring the intracellular pharmacokinetics of the 5-fluorouracil nucleotides during capecitabine treatment. Br J Clin Pharmacol. 2016;81(5):949–57.
Diasio RB, Harris BE. Clinical pharmacology of 5-fluorouracil. Clin Pharmacokinet. 1989;16(4):215–37.
Diasio RB. Clinical implications of dihydropyrimidine dehydrogenase on 5-FU pharmacology. Oncology (Williston Park). 2001;15(1 Suppl 2):21–6 (discussion 7).
Jacobs BAW, Deenen MJ, Joerger M, Rosing H, de Vries N, Meulendijks D, et al. Pharmacokinetics of capecitabine and four metabolites in a heterogeneous population of cancer patients: a comprehensive analysis. CPT Pharmacometrics Syst Pharmacol. 2019;8(12):940–50.
Shafiei M, Yoon R, McLachlan A, Boddy A, Beale P, Blinman P. Pharmacokinetics of anticancer drugs used in treatment of older adults with colorectal cancer: a systematic review. Ther Drug Monit. 2019;41(5):553–60.
d’Esposito F, Nebot N, Edwards RJ, Murray M. Impaired irinotecan biotransformation in hepatic microsomal fractions from patients with chronic liver disease. Br J Clin Pharmacol. 2010;70(3):400–8.
Twelves C, Glynne-Jones R, Cassidy J, Schüller J, Goggin T, Roos B, et al. Effect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolites. Clin Cancer Res. 1999;5(7):1696–702.
Kobuchi S, Ito Y. Application of pharmacometrics of 5-fluorouracil to personalized medicine: a tool for predicting pharmacokinetic-pharmacodynamic/toxicodynamic responses. Anticancer Res. 2020;40(12):6585–97.
Saez-Bello M, Mangas-Sanjuan V, Martinez-Gomez MA, Lopez-Montenegro Soria MA, Climente-Marti M, Merino-Sanjuan M. Evaluation of ABC gene polymorphisms on the pharmacokinetics and pharmacodynamics of capecitabine in colorectal patients: implications for dosing recommendations. Br J Clin Pharmacol. 2021;87(3):905–15.
Arshad U, Ploylearmsaeng SA, Karlsson MO, Doroshyenko O, Langer D, Schomig E, et al. Prediction of exposure-driven myelotoxicity of continuous infusion 5-fluorouracil by a semi-physiological pharmacokinetic-pharmacodynamic model in gastrointestinal cancer patients. Cancer Chemother Pharmacol. 2020;85(4):711–22.
Sakai S, Kobuchi S, Ito Y, Sakaeda T. A physiologically based pharmacokinetic-pharmacodynamic model for capecitabine in colorectal cancer rats: simulation of antitumor efficacy at various administration schedules. Eur J Drug Metab Pharmacokinet. 2021;46(2):301–15.
Tsukamoto Y, Kato Y, Ura M, Horii I, Ishitsuka H, Kusuhara H, et al. A physiologically based pharmacokinetic analysis of capecitabine, a triple prodrug of 5-FU, in humans: the mechanism for tumor-selective accumulation of 5-FU. Pharm Res. 2001;18(8):1190–202.
Tsukamoto Y, Kato Y, Ura M, Horii I, Ishikawa T, Ishitsuka H, et al. Investigation of 5-FU disposition after oral administration of capecitabine, a triple-prodrug of 5-FU, using a physiologically based pharmacokinetic model in a human cancer xenograft model: comparison of the simulated 5-FU exposures in the tumour tissue between human and xenograft model. Biopharm Drug Dispos. 2001;22(1):1–14.
Sakai S, Kobuchi S, Ito Y, Sakaeda T. Assessment of pharmacokinetic variations of capecitabine after multiple administration in rats: a physiologically based pharmacokinetic model. Cancer Chemother Pharmacol. 2020;85(5):869–80.
Sakai S, Kobuchi S, Ito Y, Sakaeda T. Assessment of drug-drug interaction and optimization in capecitabine and irinotecan combination regimen using a physiologically based pharmacokinetic model. J Pharm Sci. 2022;111(5):1522–30.
Wojtyniak JG, Britz H, Selzer D, Schwab M, Lehr T. Data digitizing: accurate and precise data extraction for quantitative systems pharmacology and physiologically-based pharmacokinetic modeling. CPT Pharmacometrics Syst Pharmacol. 2020;9(6):322–31.
Morsman JM, Sludden J, Ameyaw MM, Githang AJ, Indalo A, Ofori-Adjei D, et al. Evaluation of dihydropyrimidine dehydrogenase activity in South-west Asian, Kenyan and Ghanaian populations. Br J Clin Pharmacol. 2000;50(3):269–72.
Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;47(8):2203–6.
Edginton AN, Willmann S. Physiology-based simulations of a pathological condition: prediction of pharmacokinetics in patients with liver cirrhosis. Clin Pharmacokinet. 2008;47(11):743–52.
Prasad B, Bhatt DK, Johnson K, Chapa R, Chu X, Salphati L, et al. Abundance of phase 1 and 2 drug-metabolizing enzymes in alcoholic and hepatitis C cirrhotic livers: a quantitative targeted proteomics study. Drug Metab Dispos. 2018;46(7):943–52.
Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, Negro V, et al. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis. 2014;46(6):556–60.
Almersjo OE, Gustavsson BG, Regardh CG, Wahlen P. Pharmacokinetic studies of 5-fluorouracil after oral and intravenous administration in man. Acta Pharmacol Toxicol (Copenh). 1980;46(5):329–36.
Eissing T, Kuepfer L, Becker C, Block M, Coboeken K, Gaub T, et al. A computational systems biology software platform for multiscale modeling and simulation: integrating whole-body physiology, disease biology, and molecular reaction networks. Front Physiol. 2011;2:4.
Judson IR, Beale PJ, Trigo JM, Aherne W, Crompton T, Jones D, et al. A human capecitabine excretion balance and pharmacokinetic study after administration of a single oral dose of 14C-labelled drug. Invest New Drugs. 1999;17(1):49–56.
Palmirotta R, Lovero D, Delacour H, Le Roy A, Cremades S, Silvestris F. Rare dihydropyrimidine dehydrogenase variants and toxicity by floropyrimidines: a case report. Front Oncol. 2019;9:139.
Joel SP, Papamichael D, Richards F, Davis T, Aslanis V, Chatelut E, et al. Lack of pharmacokinetic interaction between 5-fluorouracil and oxaliplatin. Clin Pharmacol Ther. 2004;76(1):45–54.
Faugeras L, Dili A, Druez A, Krug B, Decoster C, D’Hondt L. Treatment options for metastatic colorectal cancer in patients with liver dysfunction due to malignancy. Crit Rev Oncol Hematol. 2017;115:59–66.
Fan Y, Mansoor N, Ahmad T, Khan RA, Czejka M, Sharib S, et al. Physiologically based pharmacokinetic modeling for predicting irinotecan exposure in human body. Oncotarget. 2017;8(29):48178–85.
Blesch KS, Gieschke R, Tsukamoto Y, Reigner BG, Burger HU, Steimer JL. Clinical pharmacokinetic/pharmacodynamic and physiologically based pharmacokinetic modeling in new drug development: the capecitabine experience. Invest New Drugs. 2003;21(2):195–223.
Cariello R, Federico A, Sapone A, Tuccillo C, Scialdone VR, Tiso A, et al. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig Liver Dis. 2010;42(3):200–4.
Gerner B, Scherf-Clavel O. Physiologically based pharmacokinetic modelling of cabozantinib to simulate enterohepatic recirculation, drug-drug interaction with rifampin and liver impairment. Pharmaceutics. 2021;13(6):778.
Ponziani FR, Valenza V, Nure E, Bianco G, Marrone G, Grieco A, et al. Effect of liver transplantation on intestinal permeability and correlation with infection episodes. PLoS ONE. 2020;15(6):e0235359.
Morel A, Boisdron-Celle M, Fey L, Soulie P, Craipeau MC, Traore S, et al. Clinical relevance of different dihydropyrimidine dehydrogenase gene single nucleotide polymorphisms on 5-fluorouracil tolerance. Mol Cancer Ther. 2006;5(11):2895–904.
Hishinuma E, Narita Y, Obuchi K, Ueda A, Saito S, Tadaka S, et al. Importance of rare DPYD genetic polymorphisms for 5-fluorouracil therapy in the japanese population. Front Pharmacol. 2022;13:930470.
Saif MW, Ezzeldin H, Vance K, Sellers S, Diasio RB. DPYD*2A mutation: the most common mutation associated with DPD deficiency. Cancer Chemother Pharmacol. 2007;60(4):503–7.
van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40(7):939–50.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China [grant numbers 81973394].
Author information
Authors and Affiliations
Contributions
Yu Wang collected data, conducted analysis, develop the model and wrote the manuscript; Haihong Hu collected data and conducted analysis; Su Zeng and Lushan Yu critically revised the manuscript; Su Zeng also contributed to conception design.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Hu, H., Yu, L. et al. Physiologically Based Pharmacokinetic Modeling for Prediction of 5-FU Pharmacokinetics in Cancer Patients with Hepatic Impairment After 5-FU and Capecitabine Administration. Pharm Res 40, 2177–2194 (2023). https://doi.org/10.1007/s11095-023-03585-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03585-y