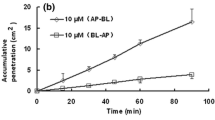
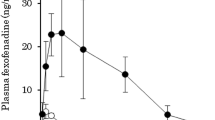
Abstract
Purpose
We aimed to compare the effects of P-glycoprotein (ABCB1) on the intestinal uptake of tenofovir disoproxil fumarate (TDF), tenofovir alafenamide fumarate (TAF), and metabolites, tenofovir isoproxil monoester (TEM) and tenofovir (TFV), and to study the molecular mechanism of drug-drug interaction (DDI) between sofosbuvir (SOF) and TDF/TAF.
Methods
Bidirectional transport experiments in Caco-2 cells and accumulation studies in precision-cut intestinal slices prepared from the ileal segment of rodent (rPCIS) and human (hPCIS) intestines were performed.
Results
TDF and TAF were extensively metabolised but TAF exhibited greater stability. ABCB1 significantly reduced the intestinal transepithelial transfer and uptake of the TFV(TDF) and TFV(TAF)-equivalents. However, TDF and TAF were absorbed more efficiently than TFV and TEM. SOF did not inhibit intestinal efflux of TDF and TAF or affect intestinal accumulation of TFV(TDF) and TFV(TAF)-equivalents but did significantly increase the proportion of absorbed TDF.
Conclusions
TDF and TAF likely produce comparable concentrations of TFV-equivalents in the portal vein and the extent of permeation is reduced by the activity of ABCB1. DDI on ABCB1 can thus potentially affect TDF and TAF absorption. SOF does not inhibit ABCB1-mediated transport of TDF and TAF but does stabilise TDF, albeit without affecting the quantity of TFV(TDF)-equivalents crossing the intestinal barrier. Our data thus suggest that reported increases in the TFV plasma concentrations in patients treated with SOF and TDF result either from a DDI between SOF and TDF that does not involve ABCB1 or from a DDI involving another drug used in combination therapy.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gilead Sciences: VIREAD®. https://www.gilead.com/-/media/files/pdfs/medicines/hiv/viread/viread_pi.pdf (2019). Accessed 22-09-2022.
Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antiviral Res. 2016;125:63–70. https://doi.org/10.1016/j.antiviral.2015.11.009.
Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9):595–612. https://doi.org/10.2165/00003088-200443090-00003.
Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45(10):2733–9. https://doi.org/10.1128/AAC.45.10.2733-2739.2001.
Van Gelder J, Shafiee M, De Clercq E, Penninckx F, Van den Mooter G, Kinget R, et al. Species-dependent and site-specific intestinal metabolism of ester prodrugs. International Journal of Pharmaceutics. 2000;205(1-2):93–100. https://doi.org/10.1016/s0378-5173(00)00507-x.
Naesens L, Bischofberger N, Augustijns P, Annaert P, Van den Mooter G, Arimilli MN, et al. Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl) adenine in mice. Antimicrob Agents Chemother. 1998;42(7):1568–73. https://doi.org/10.1128/AAC.42.7.1568.
Shailender J, Ravi PR, Saha P, Myneni S. Oral pharmacokinetic interaction of ester rich fruit juices and pharmaceutical excipients with tenofovir disoproxil fumarate in male Wistar rats. Xenobiotica. 2017;47(12):1104–11. https://doi.org/10.1080/00498254.2016.1269375.
Shen Y, Yan B. Covalent inhibition of carboxylesterase-2 by sofosbuvir and its effect on the hydrolytic activation of tenofovir disoproxil. J Hepatol. 2017;66(3):660–1. https://doi.org/10.1016/j.jhep.2016.11.025.
Brooks KM, Castillo-Mancilla JR, Blum J, Huntley R, MaWhinney S, Alexander K, et al. Increased tenofovir monoester concentrations in patients receiving tenofovir disoproxil fumarate with ledipasvir/sofosbuvir. J Antimicrob Chemother. 2019;74(8):2360–4. https://doi.org/10.1093/jac/dkz184.
Shen Y, Yan B. Inhibition of carboxylesterase 2 (CES2) by sofosbuvir: Metabolism-reduced potency, in vivo inhibition and reduced activation of the anti-HIV drug tenofovir disoproxil. Drug Metabolism and Pharmacokinetics. 2018;33(1) https://doi.org/10.1016/j.dmpk.2017.11.182.
Murphy RA, Valentovic MA. Factors Contributing to the Antiviral Effectiveness of Tenofovir. J Pharmacol Exp Ther. 2017;363(2):156–63. https://doi.org/10.1124/jpet.117.243139.
European Medicines Agency: Vemlidy Assessment report. 2016. https://www.ema.europa.eu/en/documents/assessment-report/vemlidy-epar-public-assessment-report_en.pdf. Accessed 08-09-2022.
Birkus G, Wang R, Liu X, Kutty N, MacArthur H, Cihlar T, et al. Cathepsin A is the major hydrolase catalyzing the intracellular hydrolysis of the antiretroviral nucleotide phosphonoamidate prodrugs GS-7340 and GS-9131. Antimicrob Agents Chemother. 2007;51(2):543–50. https://doi.org/10.1128/AAC.00968-06.
Birkus G, Bam RA, Willkom M, Frey CR, Tsai L, Stray KM, et al. Intracellular Activation of Tenofovir Alafenamide and the Effect of Viral and Host Protease Inhibitors. Antimicrob Agents Chemother. 2016;60(1):316–22. https://doi.org/10.1128/AAC.01834-15.
Murakami E, Wang T, Park Y, Hao J, Lepist EI, Babusis D, et al. Implications of efficient hepatic delivery by tenofovir alafenamide (GS-7340) for hepatitis B virus therapy. Antimicrob Agents Chemother. 2015;59(6):3563–9. https://doi.org/10.1128/AAC.00128-15.
Lee WA, He GX, Eisenberg E, Cihlar T, Swaminathan S, Mulato A, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother. 2005;49(5):1898–906. https://doi.org/10.1128/AAC.49.5.1898-1906.2005.
Callebaut C, Stepan G, Tian Y, Miller MD. In Vitro Virology Profile of Tenofovir Alafenamide, a Novel Oral Prodrug of Tenofovir with Improved Antiviral Activity Compared to That of Tenofovir Disoproxil Fumarate. Antimicrob Agents Chemother. 2015;59(10):5909–16. https://doi.org/10.1128/AAC.01152-15.
Orkin C, Molina J-M, Negredo E, Arribas JR, Gathe J, Eron JJ, et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. The Lancet HIV. 2018;5(1):e23–34. https://doi.org/10.1016/s2352-3018(17)30179-0.
Sax PE, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet. 2015;385(9987):2606–15. https://doi.org/10.1016/S0140-6736(15)60616-X.
Mills A, Arribas JR, Andrade-Villanueva J, DiPerri G, Van Lunzen J, Koenig E, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16(1):43–52. https://doi.org/10.1016/S1473-3099(15)00348-5.
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–99. https://doi.org/10.1002/hep.29800.
Department of Health and Human Services: Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2022. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf. Accessed 06-09-2022.
Tong L, Phan TK, Robinson KL, Babusis D, Strab R, Bhoopathy S, et al. Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrob Agents Chemother. 2007;51(10):3498–504. https://doi.org/10.1128/AAC.00671-07.
Gilead Sciences: VEMLIDY®. 2021. https://www.gilead.com/-/media/files/pdfs/medicines/liver-disease/vemlidy/vemlidy_pi.pdf. Accessed 22-09-2022.
Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52(12):1788–95. https://doi.org/10.1136/gut.52.12.1788.
Hartter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate (Pradaxa((R)) ) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75(4):1053–62. https://doi.org/10.1111/j.1365-2125.2012.04453.x.
Westphal K, Weinbrenner A, Giessmann T, Stuhr M, Franke G, Zschiesche M, et al. Oral bioavailability of digoxin is enhanced by talinolol: evidence for involvement of intestinal P-glycoprotein. Clin Pharmacol Ther. 2000;68(1):6–12. https://doi.org/10.1067/mcp.2000.107579.
Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. https://doi.org/10.1016/S1473-3099(15)00485-5.
AASLD/IDS HCV: HCV Guidance: Recommendations for testing, managing, and treating hepatitis C. 2021. https://www.hcvguidelines.org/. Accessed 08-09-2022.
MacBrayne CE, Marks KM, Fierer DS, Naggie S, Chung RT, Hughes MD, et al. Effects of sofosbuvir-based hepatitis C treatment on the pharmacokinetics of tenofovir in HIV/HCV-coinfected individuals receiving tenofovir disoproxil fumarate. J Antimicrob Chemother. 2018;73(8):2112–9. https://doi.org/10.1093/jac/dky146.
Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773–80. https://doi.org/10.1053/j.ajkd.2011.01.022.
Hamzah L, Jose S, Booth JW, Hegazi A, Rayment M, Bailey A, et al. Treatment-limiting renal tubulopathy in patients treated with tenofovir disoproxil fumarate. J Infect. 2017;74(5):492–500. https://doi.org/10.1016/j.jinf.2017.01.010.
Gilead Sciences: SOVALDI®. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204671s002lbl.pdf. Accessed 08-09-2022.
Li M, de Graaf IA, de Jager MH, Groothuis GM. P-gp activity and inhibition in the different regions of human intestine ex vivo. Biopharm Drug Dispos. 2017;38(2):127–38. https://doi.org/10.1002/bdd.2047.
de Graaf IA, Olinga P, de Jager MH, Merema MT, de Kanter R, van de Kerkhof EG, et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat Protoc. 2010;5(9):1540–51. https://doi.org/10.1038/nprot.2010.111.
Kalgutkar AS, Frederick KS, Chupka J, Feng B, Kempshall S, Mireles RJ, et al. N-(3,4-dimethoxyphenethyl)-4-(6,7-dimethoxy-3,4-dihydroisoquinolin-2[1H]-yl)-6,7- dimethoxyquinazolin-2-amine (CP-100,356) as a "chemical knock-out equivalent" to assess the impact of efflux transporters on oral drug absorption in the rat. J Pharm Sci. 2009;98(12):4914–27. https://doi.org/10.1002/jps.21756.
Dantzig AH, Shepard RL, Cao J, Law KL, Ehlhardt WJ, Baughman TM, et al. Reversal of P-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996;56(18):4171–9.
Weidner LD, Zoghbi SS, Lu S, Shukla S, Ambudkar SV, Pike VW, et al. The Inhibitor Ko143 Is Not Specific for ABCG2. J Pharmacol Exp Ther. 2015;354(3):384–93. https://doi.org/10.1124/jpet.115.225482.
Huliciak M, Vokral I, Holas O, Martinec O, Staud F, Cerveny L. Evaluation of the Potency of Anti-HIV and Anti-HCV Drugs to Inhibit P-Glycoprotein Mediated Efflux of Digoxin in Caco-2 Cell Line and Human Precision-Cut Intestinal Slices. Pharmaceuticals (Basel). 2022;15(2) https://doi.org/10.3390/ph15020242.
Hubatsch I, Ragnarsson EG, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc. 2007;2(9):2111–9. https://doi.org/10.1038/nprot.2007.303.
Hellinger E, Veszelka S, Toth AE, Walter F, Kittel A, Bakk ML, et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur J Pharm Biopharm. 2012;82(2):340–51. https://doi.org/10.1016/j.ejpb.2012.07.020.
Perloff ES, Duan SX, Skolnik PR, Greenblatt DJ, von Moltke LL. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33(6):764–70. https://doi.org/10.1124/dmd.104.002931.
Martinec O, Huliciak M, Staud F, Cecka F, Vokral I, Cerveny L. Anti-HIV and Anti-Hepatitis C Virus Drugs Inhibit P-Glycoprotein Efflux Activity in Caco-2 Cells and Precision-Cut Rat and Human Intestinal Slices. Antimicrob Agents Chemother. 2019;63(11) https://doi.org/10.1128/AAC.00910-19.
Fical L, Khalikova M, Kocova Vlckova H, Lhotska I, Hadysova Z, Vokral I, et al. Determination of Antiviral Drugs and Their Metabolites Using Micro-Solid Phase Extraction and UHPLC-MS/MS in Reversed-Phase and Hydrophilic Interaction Chromatography Modes. Molecules. 2021;26(8) https://doi.org/10.3390/molecules26082123.
Babusis D, Phan TK, Lee WA, Watkins WJ, Ray AS. Mechanism for effective lymphoid cell and tissue loading following oral administration of nucleotide prodrug GS-7340. Mol Pharm. 2013;10(2):459–66. https://doi.org/10.1021/mp3002045.
van Gelder J, Deferme S, Naesens L, De Clercq E, van den Mooter G, Kinget R, et al. Intestinal absorption enhancement of the ester prodrug tenofovir disoproxil fumarate through modulation of the biochemical barrier by defined ester mixtures. Drug Metab Dispos. 2002;30(8):924–30. https://doi.org/10.1124/dmd.30.8.924.
Brooks KM, Castillo-Mancilla JR, Morrow M, MaWhinney S, Blum J, Wyles DL, et al. Pharmacokinetics and renal safety of tenofovir alafenamide with boosted protease inhibitors and ledipasvir/sofosbuvir. J Antimicrob Chemother. 2020;75(11):3303–10. https://doi.org/10.1093/jac/dkaa299.
Srinivasan B, Kolli AR, Esch MB, Abaci HE, Shuler ML, Hickman JJ. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20(2):107–26. https://doi.org/10.1177/2211068214561025.
Olander M, Wisniewski JR, Matsson P, Lundquist P, Artursson P. The Proteome of Filter-Grown Caco-2 Cells With a Focus on Proteins Involved in Drug Disposition. J Pharm Sci. 2016;105(2):817–27. https://doi.org/10.1016/j.xphs.2015.10.030.
Bruck S, Strohmeier J, Busch D, Drozdzik M, Oswald S. Caco-2 cells - expression, regulation and function of drug transporters compared with human jejunal tissue. Biopharm Drug Dispos. 2017;38(2):115–26. https://doi.org/10.1002/bdd.2025.
Imai T, Imoto M, Sakamoto H, Hashimoto M. Identification of esterases expressed in Caco-2 cells and effects of their hydrolyzing activity in predicting human intestinal absorption. Drug Metab Dispos. 2005;33(8):1185–90. https://doi.org/10.1124/dmd.105.004226.
Li M, de Graaf IA, Groothuis GM. Precision-cut intestinal slices: alternative model for drug transport, metabolism, and toxicology research. Expert Opin Drug Metab Toxicol. 2016;12(2):175–90. https://doi.org/10.1517/17425255.2016.1125882.
Martinec O, Biel C, de Graaf IAM, Huliciak M, de Jong KP, Staud F, et al. Rifampicin Induces Gene, Protein, and Activity of P-Glycoprotein (ABCB1) in Human Precision-Cut Intestinal Slices. Front Pharmacol. 2021;12:684156. https://doi.org/10.3389/fphar.2021.684156.
Taketani M, Shii M, Ohura K, Ninomiya S, Imai T. Carboxylesterase in the liver and small intestine of experimental animals and human. Life Sci. 2007;81(11):924–32. https://doi.org/10.1016/j.lfs.2007.07.026.
Moss DM, Domanico P, Watkins M, Park S, Randolph R, Wring S, et al. Simulating Intestinal Transporter and Enzyme Activity in a Physiologically Based Pharmacokinetic Model for Tenofovir Disoproxil Fumarate. Antimicrob Agents Chemother. 2017;61(7) https://doi.org/10.1128/AAC.00105-17.
Li J, Liu S, Shi J, Zhu HJ. Activation of Tenofovir Alafenamide and Sofosbuvir in the Human Lung and Its Implications in the Development of Nucleoside/Nucleotide Prodrugs for Treating SARS-CoV-2 Pulmonary Infection. Pharmaceutics. 2021;13(10) https://doi.org/10.3390/pharmaceutics13101656.
Neumanova Z, Cerveny L, Ceckova M, Staud F. Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta. AIDS. 2014;28(1):9–17. https://doi.org/10.1097/QAD.0000000000000112.
Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50(10):3297–304. https://doi.org/10.1128/AAC.00251-06.
Li J, Shi J, Xiao J, Tran L, Wang X, Zhu HJ. Contributions of Cathepsin A and Carboxylesterase 1 to the Hydrolysis of Tenofovir Alafenamide in the Human Liver, and the Effect of CES1 Genetic Variation on Tenofovir Alafenamide Hydrolysis. Drug Metab Dispos. 2022;50(3):243–8. https://doi.org/10.1124/dmd.120.000323.
Satoh T, Hosokawa M. Structure, function and regulation of carboxylesterases. Chem Biol Interact. 2006;162(3):195–211. https://doi.org/10.1016/j.cbi.2006.07.001.
Takayama K, Ito K, Matsui A, Yamashita T, Kawakami K, Hirayama D, et al. In Vivo Gene Expression Profile of Human Intestinal Epithelial Cells: From the Viewpoint of Drug Metabolism and Pharmacokinetics. Drug Metab Dispos. 2021;49(3):221–32. https://doi.org/10.1124/dmd.120.000283.
Mai Y, Dou L, Yao Z, Madla CM, Gavins FKH, Taherali F, et al. Quantification of P-Glycoprotein in the Gastrointestinal Tract of Humans and Rodents: Methodology, Gut Region, Sex, and Species Matter. Mol Pharm. 2021;18(5):1895–904. https://doi.org/10.1021/acs.molpharmaceut.0c00574.
Dahan A, Amidon GL. Segmental Dependent Transport of Low Permeability Compounds along the Small Intestine Due to P-Glycoprotein: The Role of Efflux Transport in the Oral Absorption of BCS Class III Drugs. Mol Pharmaceut. 2009;6(1):19–28. https://doi.org/10.1021/mp800088f.
Patil S, Kadam C, Pokharkar V. QbD based approach for optimization of Tenofovir disoproxil fumarate loaded liquid crystal precursor with improved permeability. J Adv Res. 2017;8(6):607–16. https://doi.org/10.1016/j.jare.2017.07.005.
Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review(s) Ledipasvir/Sofosbuvir. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205834Orig1s000ClinPharmR.pdf. Accessed 10-09-2022.
US Food and Drug Administration: In Vitro Drug Interaction Studies - Cytochrome P450 Enzyme and Transporter Mediated Drug Interactions. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/in-vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions. Accessed 20-09-2022.
Geboers S, Haenen S, Mols R, Brouwers J, Tack J, Annaert P, et al. Intestinal behavior of the ester prodrug tenofovir DF in humans. Int J Pharm. 2015;485(1-2):131–7. https://doi.org/10.1016/j.ijpharm.2015.03.002.
Golla VM, Kurmi M, Shaik K, Singh S. Stability behaviour of antiretroviral drugs and their combinations. 4: Characterization of degradation products of tenofovir alafenamide fumarate and comparison of its degradation and stability behaviour with tenofovir disoproxil fumarate. J Pharm Biomed Anal. 2016;131:146–55. https://doi.org/10.1016/j.jpba.2016.08.022.
Aungst BJ. The Effects of Dose Volume and Excipient Dose on Luminal Concentration and Oral Drug Absorption. AAPS J. 2020;22(5):99. https://doi.org/10.1208/s12248-020-00490-9.
Acknowledgements
We thank Gilead Sciences for providing TDF and TEM. We also thank all donors of ileal samples.
Funding
This study was supported by a research grant from Czech Science Foundation No. GACR 18-07281Y, Charles University grant Agency No. GAUK 364521, and project SVV No. 260 663. The analyses were performed on instruments purchased from the project EFSA-CDN (reg. no.: CZ.02.1.01/0.0/0.0/16_019/0000841) co-funded by ERDF.
Author information
Authors and Affiliations
Contributions
Martin Huliciak: Writing - Original Draft, Methodology, Validation, Investigation, Data Curation, Formal analysis, Resources, Visualization, Project administration, Funding acquisition. Ivona Lhotska: Methodology, Validation, Investigation, Data Curation. Hana Kocova-Vlckova: Methodology, Validation, Resources. Veronika Halodova: Investigation. Tomas Dusek: Resources. Filip Cecka: Resources. Frantisek Staud: Funding acquisition. Ivan Vokral: Conceptualization, Methodology, Validation, Investigation, Resources, Supervision, Writing - Review & Editing, Funding acquisition. Lukas Cerveny: Conceptualization, Supervision, Project administration, Methodology, Writing-Original Draft, Writing - Review & Editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huliciak, M., Lhotska, I., Kocova-Vlckova, H. et al. Effect of P-glycoprotein and Cotreatment with Sofosbuvir on the Intestinal Permeation of Tenofovir Disoproxil Fumarate and Tenofovir Alafenamide Fumarate. Pharm Res 40, 2109–2120 (2023). https://doi.org/10.1007/s11095-023-03581-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03581-2