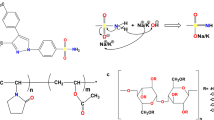
Abstract
Objectives
Amorphous solid dispersions (ASDs), wherein a drug is molecularly dispersed in a polymer, can improve physical stability and oral bioavailability of poorly soluble drugs. Risk of drug crystallization is usually averted using high polymer concentrations. However, we demonstrated recently that the overlap concentration, C*, of polymer in drug melt is the minimum polymer concentration required to maintain drug in the amorphous state following rapid quench. This conclusion was confirmed for several drugs mixed with poly(vinylpyrrolidone) (PVP). Here we assess the solid-state stability of ASDs formulated with a variety of polymers and drugs and at various polymer concentrations (C) and molecular weights (MWs). We further test the hypothesis that degree of drug crystallization decreases with increasing C/C* and vanishes when C>C*, where C* depends on polymer MW and strength of drug-polymer interaction.
Methods
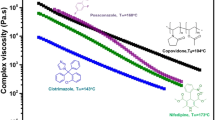
We test our hypothesis with ASDs consisting of ketoconazole admixed with polyacrylic acid, polymethacrylic acid and poly (methacrylic acid-co-ethyl acrylate); and felodipine admixed with PVP and poly (vinylpyrrolidone-co-vinyl acetate). Values of C* for polymers in molten drug are rheologically determined. Crystallization behavior is assessed by measuring enthalpy of fusion, ΔHf and by X-ray diffraction.
Results
We confirm that ΔHf/ΔHf, C = 0 = f(C/C∗), and essentially no crystallization occurs when C>C*.
Conclusions
Our findings will aid researchers in designing or selecting appropriate polymers to inhibit crystallization of poorly soluble drugs. This research also suggests that C* as determined by rheology can be used to compare drug-polymer interactions for similar molecular weight polymers.
Graphical abstract








Similar content being viewed by others
References
Sahoo A, Suryanarayanan R, Siegel RA. Stabilization of amorphous drugs by polymers: The role of overlap concentration (C*). Mol Pharm. 2020; 17:4401-4406
Sahoo A, Kumar NSK, Suryanarayanan, R. Crosslinking: An avenue to develop stable amorphous solid dispersion with high drug loading and tailored physical stability. J Control Release. 2019; 311-312:212-224.
Di L, Fish PV, Mano T. Bridging solubility between drug discovery and development. Drug Discov Today 2012; 17:486-495.
Janssens S, Van der Mooter G. Review: Physical chemistry of solid dispersions. J Pharm Pharmacol 2009; 61:1571-1586.
Luebbert C, Huxoll F, Sadowski G. Amorphous-amorphous phase separation in API/polymer formulations. Molecules 2017; 22:296.
Tian B, Tang X, Taylor LS. Investigating the correlation between miscibility and physical stability of amorphous solid dispersions using fluorescence-based techniques. Mol Pharm 2016; 13:3988-4000.
Meng F, Trivino A, Prasad D, Chauhan H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur J Pharm Sci 2015; 71:12-24.
Jog R, Gokhale R, Burgess DJ. Solid state drug-polymer miscibility studies using the model drug ABT-102. Int J Pharm 2016; 509:285-295.
de Gennes PG. Scaling Concepts in Polymer Physics. Cornell University Press; 1979.
Song S, Wang C, Zhang B, Sun CC, Lodge TP, Siegel, RA. A rheological approach for predicting the physical stability of amorphous solid dispersions. J Pharm Sci 2023; 112:204-212.
Sahoo A, Jassal M, Agrawal AK. Discovery of unique dual response in acrylonitrile polymers. Smart Mater Struct 2007; 16:1843-1848.
Hiemenz PC, Lodge TP. Polymer Chemistry, 2nd ed. CRC Press; 2007.
Graessley WW. Polymer chain dimensions and dependence of viscoelastic properties on concentration, molecular weight, and solvent power. Polymer 1980; 21:258-262.
Colby RH, Rubinstein M. Two-parameter scaling for polymers in Θ solvents. Macromolecules 1990; 23:2753-2757.
Lopex-Cebral R, Martin-Pastor M, Paolicelli P, Casadei MA, Seijo B, Sanchez A. Application of NMR spectroscopy in the development of biomimetic approach for hydrophobic drug association with physical hydrogels. Coll Surf B: Biointerfaces 2014; 115:391-399.
Whitehouse LW, Menzies A, Dawson, B. Mouse hepatic metabolites of ketoconazole: Isolation and structure elucidation. J Pharm Biomed Anal 1994; 12:1425.
Redenti E, Ventura P, Fronza G. Experimental and theoretical analysis of the interaction of (±)-cis-ketoconazole with β-cyclodextrin in the presence of (+)-L-tartaric acid. J Pharm Sci 1999; 88:599-607.
Handbook of Proton NMR Spectra and Data. Elsevier; 1987.
Fielding L. Determination of association constants (Ka) from solution NMR data. Tetrahedron 2000; 56:6151−6170.
Sarpal K, Delaney S, Zhang GGZ, Munson EJ. Phase behavior of amorphous solid dispersion of felodipine: homogeneity and drug polymer interactions. Mol Pharm 2019; 16:4836-4851.
Tanaka R, Hattori Y, Horie H, Kamada H, Nagato T, Otsuka M. Characterization of amorphous solid dispersion of pharmaceutical compound with pH-dependent solubility prepared by continuous-spray granulator. Pharmaceutics 2019; 11:159.
Maurer JJ, Eustace DJ, Ratcliffe CT. Thermal characterization of poly (acrylic acid). Macromolecules 1987; 20:196-202.
Kumar YM, Kayyarapu B, Neeuganati OG, Chekuri R. Thermal and conductivity studies of VO2+ doped methacrylic acid-ethyl acrylate (MAA: EA) copolymer film. Mater Res 2018;21: e20170328.
Acknowledgments
Work was funded by Industrial Partners for Research in Interfacial and Materials Engineering (IPRIME), an academic-industrial consortium at the University of Minnesota (UMN). We thank Profs. Christopher Macosko and Christopher Ellison, Department of Chemical Engineering and Material Science, UMN, for providing access to their microcompounder, rheometer, and SEC. Portions of this work were carried out in the Characterization Facility, UMN, which receives partial support from NSF through the MRSEC program.
Supplementary Information
DSC curves for ASDs prepared with PVP polymers and drug (FEL and KTZ), Proton NMR data for PAA and MAE based ASDs, VTXRD data for MAE and PAA (HMW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Financial Interest
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1.78 mb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahoo, A., Siegel, R.A. Drug-Polymer Miscibility and the Overlap Concentration (C*) as Measured by Rheology: Variation of Polymer Structure. Pharm Res 40, 2229–2237 (2023). https://doi.org/10.1007/s11095-023-03570-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03570-5