Abstract
Background
Understanding the impact of altered hepatic uptake and/or efflux on the hepatobiliary disposition of the imaging agents [99mTc]Mebrofenin (MEB) and [153Gd]Gadobenate dimeglumine (BOPTA) is important for proper estimation of liver function.
Methods
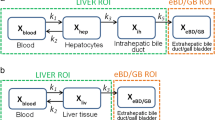
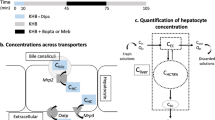
A multi-compartmental pharmacokinetic (PK) model describing MEB and BOPTA disposition in isolated perfused rat livers (IPRLs) was developed. The PK model was simultaneously fit to MEB and BOPTA concentration-time data in the extracellular space, hepatocytes, bile canaliculi, and sinusoidal efflux in livers from healthy rats, and to BOPTA concentration-time data in rats pretreated with monocrotaline (MCT).
Results
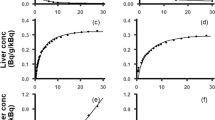
The model adequately described MEB and BOPTA disposition in each compartment. The hepatocyte uptake clearance was much higher for MEB (55.3 mL/min) than BOPTA (6.67 mL/min), whereas the sinusoidal efflux clearance for MEB (0.000831 mL/min) was lower than BOPTA (0.0127 mL/min). The clearance from hepatocytes to bile (CLbc) for MEB (0.658 mL/min) was similar to BOPTA (0.642 mL/min) in healthy rat livers. The BOPTA CLbc was reduced in livers from MCT-pretreated rats (0.496 mL/min), while the sinusoidal efflux clearance was increased (0.0644 mL/min).
Conclusion
A PK model developed to characterize MEB and BOPTA disposition in IPRLs was used to quantify changes in the hepatobiliary disposition of BOPTA caused by MCT pretreatment of rats to induce liver toxicity. This PK model could be applied to simulate changes in the hepatobiliary disposition of these imaging agents in rats in response to altered hepatocyte uptake or efflux associated with disease, toxicity, or drug-drug interactions.






Similar content being viewed by others
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Pastor CM, Mullhaupt B, Stieger B. The role of organic anion transporters in diagnosing liver diseases by magnetic resonance imaging. Drug Metab Dispos. 2014;42:675–84.
Kusuhara H. Imaging in the study of membrane transporters. Clin Pharmacol Ther. 2013;94:33–6.
Guo Y, Chu X, Parrott NJ, Brouwer KLR, Hsu V, Nagar S, et al. Advancing predictions of tissue and intracellular drug concentrations using in vitro, imaging and PBPK modeling approaches. Clin Pharmacol Ther. 2018;104:865–89.
Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, et al. Physiological and biochemical basis of clinical liver function tests. Ann Surg. 2013;257:27–36.
Giacomini, KM, Huang S, Tweedie DJ, et al. (The International Transporter Consortium). Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–36.
de Graaf W, Häusler S, Heger M, van Ginhoven TM, van Cappellen G, Bennink RJ, Kullak-Ublick GA, Hesselmann R, van Gulik TM, Stieger B. Transporters involved in the hepatic uptake of (99m)Tc-mebrofenin and indocyanine green. J Hepatol. 2011;54(4):738–45.
Shuboni-Mulligan DD, Parys M, Blanco-Fernandez B, Mallett CL, Schnegelberger R, Takada M, et al. Dynamic contrast-enhanced MRI of OATP dysfunction in diabetes. Diabetes. 2019;68(2):271–80.
Ghibellini G, Leslie EM, Pollack GM, Brouwer KLR. Use of Tc-99m mebrofenin as a clinical probe to assess altered hepatobiliary transport: integration of in vitro, pharmacokinetic modeling, and simulation studies. Pharm Res. 2008;25:1851–60.
Millet P, Moulin M, Stieger B, Daali Y, Pastor CM. How organic anions accumulate in hepatocytes lacking Mrp2: evidence in rat liver. J Pharmacol Exp Ther. 2011;336:624–32.
Planchamp C, Hadengue A, Stieger B, Bourquin J, Vonlaufen A, Frossard JL, et al. Function of both sinusoidal and canalicular transporters controls the concentration of organic anions within hepatocytes. Mol Pharmacol. 2007;71:1089–97.
Marie S, Hernández-Lozano I, Breuil L, Saba W, Novell A, Gennisson JL, et al. Validation of pharmacological protocols for targeted inhibition of canalicular MRP2 activity in hepatocytes using [99mTc]mebrofenin imaging in rats. Pharmaceutics. 2020;12(6):486.
Bhargava KK, Joseph B, Ananthanarayanan M, Balasubramaniyan N, Tronco GG, Palestro CJ, et al. Adenosine triphosphate-binding cassette subfamily C member 2 is the major transporter of the hepatobiliary imaging agent (99m)Tc-mebrofenin. J Nucl Med. 2009;50(7):1140–6.
Hernández Lozano I, Langer O. Use of imaging to assess the activity of hepatic transporters. Expert Opin Drug Metab Toxicol. 2020;16(2):149–64.
Pastor CM, Brouwer KLR. New pharmacokinetic parameters of imaging substrates quantified from rat liver compartments. Drug Metab Dispos. 2022;50(1):58–64.
Bonnaventure P, Cusin F, Pastor CM. Hepatocyte concentrations of imaging compounds associated with transporter inhibition: evidence in perfused rat livers. Drug Metab Dispos. 2019;47:412–8.
Sourbron S, Sommer WH, Reiser MF, Zech CJ. Combined quantification of liver perfusion and function with dynamic gadoxetic acid-enhanced MR imaging. Radiology. 2012;263:874–83.
Leporq B, Daire JL, Pastor CM, Deltenre P, Sempoux C, Schmidt S, et al. Quantification of hepatic perfusion and hepatocyte function with dynamic gadoxetic acid-enhanced MRI in patients with chronic liver disease. Clin Sci. 2018;132:813–24.
Poetter-Lang S, Bastati N, Messner A, Kristic A, Herold A, Hodge JC, et al. Quantification of liver function using gadoxetic acid-enhanced MRI. Abdom Radiol. 2020;45(11):3532–44.
Zhou IY, Catalano OA, Caravan P. Advances in functional and molecular MRI technologies in chronic liver diseases. J Hepatol. 2020;73:1241–54.
Marie S, Hernández-Lozano I, Le Vée M, Breuil L, Saba W, Goislard M, et al. Pharmacokinetic imaging using 99mTc-mebrofenin to untangle the pattern of hepatocyte transporter disruptions induced by endotoxemia in rats. Pharmaceuticals. 2022;15(4):392.
Pastor CM. Isolated perfused rat livers to quantify the pharmacokinetics and concentrations of Gd-BOPTA. Contrast Media Mol Imaging. 2018:3839108–3839111.
Pastor CM, Joly F, Vilgrain V, Millet P. Concentrations and pharmacokinetic parameters of MRI and SPECT hepatobiliary agents in rat liver compartments. Eur Radiol Exp. 2021;5(1):42.
Pastor CM, Vilgrain V. Monocrotaline toxicity alters the function of hepatocyte membrane transporters in rats. Int J Mol Sci. 2022;23(14):7928.
Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72:441–455.
Masyuk TV, Rittman EL, LaRusso NF. Quantitative assessment of the rat intrahepatic biliary system by three-dimensional reconstruction. Am J Pathol. 2001;158(6):2079–88.
Gupta M, Choudhury PS, Singh S, Hazarika D. Liver functional volumetry by Tc-99m mebrofenin hepatobiliary scintigraphy before major liver resection: a game changer. Indian J Nucl Med. 2018;33(4):277–283.
Guiu B, Deshayes E, Panaro F, Sanglier F, Cusumano C, Herrerro A, et al. 99mTc-mebrofenin hepatobiliary scintigraphy and volume metrics before liver preparation: correlations and discrepancies in non-cirrhotic patients. Ann Transl Med. 2021;9(9):795.
Jeong WK, Kim YK, Song KD, Choi D, Lim HK. The MR imaging diagnosis of liver diseases using gadoxetic acid: emphasis on hepatobiliary phase. Clin Mol Hepatol. 2013;19(4):360–6.
Liu C, Shen Z, Ma H, Wang X, Wang X, Liu K, et al. Gd-BOPTA-enhanced hepatobiliary phase MR imaging can predict the prognosis of patients with acute-on-chronic liver failure. Eur Radiol. 2022;32(5):3006–15.
Sherer F, Van Simaeys G, Kers J, Yuan Q, Doumont G, Laute M-A et al. Dynamic molecular imaging for hepatic function assessment in mice: evaluation in endotoxin-induced and warm ischemia-reperfusion models of acute liver failure. J Liver. 2015;4(1).
Tanaka Y, Chen C, Maher JM, Klaassen CD. Ischemia-reperfusion of rat livers decreases liver and increases kidney multidrug resistance associated protein 2 (Mrp2). Toxicol Sci. 2008;101(1):171–8.
Krishnamurthy GT, Turner FE. Pharmacokinetics and clinical application of technetium 99m-labeled hepatobiliary agents. Semin Nucl Med. 1990;20(2):130–49.
Knopp MV, Giesel FL, Radeleff J, Von Tengg-Kobligk H. Bile-tagged 3d magnetic resonance colonography after exclusive intravenous administration of gadobenate dimeglumine, a contrast agent with partial hepatobiliary excretion. Invest Radiol. 2001;36(10):619–23.
Schiffer E, Frossard JL, Rubbia-Brandt L, Mentha G, Pastor CM. Hepatic regeneration is decreased in a rat model of sinusoidal obstruction syndrome. J Surg Oncol. 2009;99:439–46.
DeLeve LD, Ito Y, Bethea NW, McCuskey MK, Wang X, McCuskey RS. Embolization by sinusoidal lining cells obstructs the microcirculation in rat sinusoidal obstruction syndrome. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1045–52.
Neuman MG, Cohen L, Opris M, Nanau RM, Hyunjin J. Hepatotoxicity of pyrrolizidine alkaloids. J Pharm Pharm Sci. 2015;18:825–43.
Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165(5):1260–87.
Fallon JK, Smith PC, Xia CQ, Kim MS. Quantification of four efflux drug transporters in liver and kidney across species using targeted quantitative proteomics by isotope dilution NanoLC-MS/MS. Pharm Res. 2016;33(9):2280–8.
Wang L, Prasad B, Salphati L, Chu X, Gupta A, Hop CE, Evers R, Unadkat JD. Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics. Drug Metab Dispos. 2015;43(3):367–74.
Acknowledgements
WinNonlin software was generously provided to the Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, by Certara as a member of the Pharsight Academic Center of Excellence Program. This work in part, was presented at the 24th International Symposium on Microsomes and Drug Oxidations (MDO)/ 13th International Meeting of the International Society for the Study of Xenobiotics (ISSX).
Funding
This research was supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Number R35 GM122576, and the Swiss National Foundation (Grant 310030-126030). Angela Jeong was supported by a Pharmacokinetics/Pharmacodynamics Fellowship from Allucent.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare that they have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jeong, A., Pastor, C.M. & Brouwer, K.L.R. Application of Pharmacokinetic Modeling to Characterize Hepatobiliary Disposition of Imaging Agents and Alterations due to Liver Injury in Isolated Perfused Rat Livers. Pharm Res 40, 2513–2523 (2023). https://doi.org/10.1007/s11095-023-03549-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03549-2