Abstract
Introduction
The screening of multicomponent crystal system (MCC) is a key method for improving physicochemical properties of active pharmaceutical ingredients (APIs). The challenges associated with experimental salt screening include a large number of potential counterions and solvent systems and tendency to undergo disproportionation to produce free form during crystallization. These challenges may be mitigated by a combination of experimental and computational approaches to salt screening. The goal of this study is to evaluate performance of the counterion screening methods and propose and validate novel approaches to virtual solvent screening for MCC crystallization.
Methods
The actual performance of the ΔpKa > 3 rule for counterion selection was validated using multiple screenings reports. Novel computational models for virtual solvent screening to avoid MCC incongruent crystallization were proposed. Using the ΔpKa rule, 10 acid counterions were selected for experimental aripiprazole (APZ) salt screening using 10 organic solvents. The experimental results were used to validate the proposed novel virtual solvent screen models.
Results
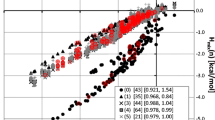
Experimental APZ salt screening resulted in a total of eight MCCs which included glucuronate, mesylate, oxalate, tartrate, salicylate and mandelate. The new model to virtually screen solvents provided a general agreement with APZ experimental findings in terms of selecting the optimal solvent for MCC crystallization.
Conclusion
The rational selection of counterions and organic solvents for MCC crystallization was presented using combined novel computational model as well as experimental studies. The current virtual solvent screen model was successfully implemented and validated which can be easily applied to newly discovered APIs.
Graphical Abstract





Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Newman A, Wenslow R. Solid form changes during drug development: good, bad, and ugly case studies. AAPS Open. 2016;2(1):2.
Aitipamula S, Banerjee R, Bansal AK, Biradha K, Cheney ML, Choudhury AR, Desiraju GR, Dikundwar AG, Dubey R, Duggirala N. Polymorphs, salts, and cocrystals: what’s in a name? Cryst Growth Des. 2012;12(5):2147–52.
Paulekuhn GS, Dressman JB, Saal C. Trends in active pharmaceutical ingredient salt selection based on analysis of the orange book database. J Med Chem. 2007;50(26):6665–72.
Morris KR, Fakes MG, Thakur AB, Newman AW, Singh AK, Venit JJ, Spagnuolo CJ, Serajuddin AT. An integrated approach to the selection of optimal salt form for a new drug candidate. Int J Pharm. 1994;105(3):209–17.
Casares AF, Nap WM, Ten Figás G, Huizenga P, Groot R, Hoffmann M. An evaluation of salt screening methodologies. J Pharm Pharmacol. 2015;67(6):812–22.
da Silva CC, Guimarães FF, Ribeiro L, Martins FT. Salt or cocrystal of salt? Probing the nature of multicomponent crystal forms with infrared spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2016;167:89–95.
Chiarella RA, Davey RJ, Peterson ML. Making co-crystals the utility of ternary phase diagrams. Cryst Growth Des. 2007;7(7):1223–6.
Computational pharmaceutical solid state chemistry. Abramov YA, editor. Wiley & Sons, Hoboken, NJ, USA, 2016.
Abramov YA, Sun G, Zeng Q. Emerging landscape of computational modeling in pharmaceutical development. J Chem Inf Model. 2022;62(5):1160–71.
Abramov YA, Loschen C, Klamt A. Rational coformer or solvent selection for pharmaceutical cocrystallization or desolvation. J Pharm Sci. 2012;101(10):3687–97.
Musumeci D, Hunter CA, Prohens R, Scuderi S, McCabe JF. Virtual cocrystal screening. Chem Sci. 2011;2(5):883–90.
Sun G, Jin Y, Li S, Yang Z, Shi B, Chang C, Abramov YA. Virtual coformer screening by crystal structure predictions: crucial role of crystallinity in pharmaceutical cocrystallization. J Phys Chem Lett. 2020;11(20):8832–8.
Yuan J, Liu X, Wang S, Chang C, Zeng Q, Song Z, Jin Y, Zeng Q, Sun G, Ruan S, Greenwell C, Abramov YA. Virtual coformer screening by a combined machine learning and physics-based approach. CrystEngComm. 2021;23(35):6039–44.
McGavin JK, Goa KL. Aripiprazole. CNS Drugs. 2002;16(11):779–786.
Cuomo A, BeccariniCrescenzi B, Goracci A, Bolognesi S, Giordano N, Rossi R, Facchi E, Neal SM, Fagiolini A. Drug safety evaluation of aripiprazole in bipolar disorder. Expert Opin Drug Saf. 2019;18(6):455–63.
Braun DE, Gelbrich T, Kahlenberg V, Tessadri R, Wieser J, Griesser UJ. Stability of solvates and packing systematics of nine crystal forms of the antipsychotic drug aripiprazole. Crys Growth Des. 2009;9(2):1054–65.
Zeidan T, Trotta J, Tilak P, Oliveira M, Chiarella R, Foxman B, Almarsson Ö, Hickey M. An unprecedented case of dodecamorphism: the twelfth polymorph of aripiprazole formed by seeding with its active metabolite. CrystEngComm. 2016;18(9):1486–8.
Zhao Y, Sun B, Jia L, Wang Y, Wang M, Yang H, Qiao Y, Gong J, Tang W. Tuning physicochemical properties of antipsychotic drug aripiprazole with multicomponent crystal strategy based on structure and property relationship. Cryst Growth Des. 2020;20(6):3747–61.
Li ZJ, Abramov YA, Bordner J, Leonard J, Medek A, Trask AV. Solid-state acid− base interactions in complexes of heterocyclic bases with dicarboxylic acids: crystallography, hydrogen bond analysis, and 15N NMR spectroscopy. J Am Chem Soc. 2006;128(25):8199–210.
Pharmaceutical salts: Properties, selection and use. Stahl PH, Wermuth CG., editors. John Wiley & Sons; 2002.
Groom CR, Bruno IJ, Lightfoot MP, Ward SC. The Cambridge structural database. Acta Crystallogr B Struct Sci Cryst Eng Mater. 2016;72(2):171–9.
Cruz-Cabeza AJ. Acid–base crystalline complexes and the pKa rule. CrystEngComm. 2012;14(20):6362–5.
Black SN, Collier EA, Davey RJ, Roberts RJ. Structure, solubility, screening, and synthesis of molecular salts. J Pharm Sci. 2007;96(5):1053–68.
Okezue M, Bogdanowich-Knipp S, Smith D, Zeller M, Byrn S, Smith P, Purcell DK, Clase K. Salts and Polymorph Screens for Bedaquiline. AAPS Pharm Sci Tech. 2021;22(7):228.
Koftis TV, Georgopoulou I, Strongilos A, Liepouri F., Panagiotidis T, Lithadioti, A. Process for the preparation of o-desmethyl-venlafaxine and salts thereof. Patent US8754261B2; 2009.
Collman BM, Miller JM, Seadeek C, Stambek JA, Blackburn AC. Comparison of a rational vs. high throughput approach for rapid salt screening and selection. Drug Dev Ind Pharm. 2013;39(1):29–38.
Remenar JF, MacPhee JM, Larson BK, Tyagi VA, Ho JH, McIlroy DA, Hickey MB, Shaw PB, Almarsson Ö. Salt selection and simultaneous polymorphism assessment via high-throughput crystallization: the case of sertraline. Org Process Res Dev. 2003;7(6):990–6.
Loschen C, Klamt A. New Developments in Prediction of Solid-State Solubility and Cocrystallization Using COSMO-RS Theory. In: Abramov YA, editor. Computational Pharmaceutical Solid State Chemistry. John Wiley and Sons; 2016. p. 211–33.
Avdeef A, Sugano K. Salt Solubility and Disproportionation-Uses and Limitations of Equations for pHmax and the In-silico Prediction of pHmax. J Pharm Sci. 2022;111(1):225–46.
BIOVIA COSMOTherm, San Diego: Dassault Systèmes; 2022.
Bento AP, Hersey A, Félix E, Landrum G, Gaulton A, Atkinson F, Bellis LJ, De Veij M, Leach AR. An open source chemical structure curation pipeline using RDKit. J Cheminform. 2020;12:1–16.
BIOVIA DMOL3, San Diego: Dassault Systèmes; 2022.
Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A. 1988;38(6):3098.
Perdew JP. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B. 1986;33(12):8822.
Perdew JP. Erratum: Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys Rev B. 1986;34(10):7406.
Andzelm J, Kölmel C, Klamt A. Incorporation of solvent effects into density functional calculations of molecular energies effects into density functional calculations of molecular energies and geometries. J Chem Phys. 1995;103(21):9312–20.
Brittain HG. Aripiprazole: polymorphs and solvatomorphs. In: Brittain HG, editor. Profiles of Drug Substances, Excipients and Related Methodology. Academic Press; vol. 37, 2012. p.1–29.
Freire E, Polla G, Baggio R. Aripiprazole salts. III. Bis (aripiprazolium) oxalate–oxalic acid (1/1). Acta Crystallogr C Struct Chem. 2013;69(2):186–90.
Zhou Q, Tan Z, Yang D, Tu J, Wang Y, Zhang Y, Liu Y, Gan G. Improving the Solubility of Aripiprazole by Multicomponent Crystallization. Crystals. 2021;11(4):343.
Acknowledgements
The authors would like to Brahma Reddy from MSN Pharmaceuticals (Hyderabad, India) for
providing us with the API sample to carry out our research work. The authors greatly thankful to J-Star Research Inc (A Porton Company) for providing us with necessary support to conduct laboratory work as well as computational work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Yuriy Abramov (YA) and Shamming Kuang (SK) were involved with idea conceptualization. YA proposed the virtual screening approaches and performed all the calculations. SK together with Harsh S. Shah (HS) designed the experimental study. Caroline Michelle (CM) performed methodology and investigations. HS was involved in data curation and formal analysis. YA and HS writing- original draft preparation, Tian Xie performed NMR data analysis and interpretation, reviewing and editing. Kaushalendra Chaturvedi assisted with methodology, lab support for experiments execution. Final review, edits and supervision was performed by SK and YA.
Corresponding authors
Ethics declarations
Conflicts of Interests/Competing Interests
The authors declare that they have no conflicts of interests/competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, H.S., Michelle, C., Xie, T. et al. Computational and Experimental Screening Approaches to Aripiprazole Salt Crystallization. Pharm Res 40, 2779–2789 (2023). https://doi.org/10.1007/s11095-023-03522-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03522-z