Abstract
Purpose
Pradofloxacin is an important antibiotic with poor physical stability. At present, there is no systematic study on its polymorphic form. The purpose of this study is to develop new crystal forms to improve the stability of Pradofloxacin and systematically study the crystal transformation relationships to guide industrial production.
Method
In this work, three solvent-free forms (Form A, Form B and Form C), a new dimethyl sulfoxide solvate (Form PL-DMSO) and a new hydrate (Form PL-H) were successfully obtained and the single crystal data of Form A, Form B and Form PL-DMSO were solved for the first time. Various solid state analysis techniques and slurry experiments have been used to evaluate the stability and determine phase transformation relationships of five crystal forms, the analysis of crystal structure provided theoretical support for the results.
Result
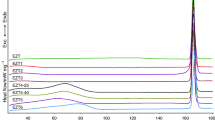
The water vapor adsorption and desorption experiences of Forms A, B, C and Form PL-H were studied, and the results show that the new hydrate has good hygroscopic stability and certain development potential. The thermal stability of different forms was determined by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) and the crystal structure shows that there are more hydrogen bonds and C − H···π interactions in form B, which is the reason why Form B is more stable than form A. Finally, the phase transformation relationships of the five crystal forms were systematically studied and discussed.
Conclusion
These results are helpful to provide guiding methods in the production and storage of pradofloxacin.















Similar content being viewed by others
Data Availability
Data generated for this study are available upon request to the corresponding author.
References
Shi Q, Chen H, Wang Y, Xu J, Liu Z, Zhang C. Recent advances in drug polymorphs: aspects of pharmaceutical properties and selective crystallization. Int J Pharm. 2022;611: 121320.
Xuan B, Wong SN, Zhang Y, Weng J, Tong HHY, Wang C, et al. Extended release of highly water soluble isoniazid attained through cocrystallization with curcumin. Cryst Growth Des. 2020;20:1951–60.
Lévesque A, Maris T, Wuest JD. ROY reclaims its crown: new ways to increase polymorphic diversity. J Am Chem Soc. 2020;142:11873–83.
Zhang Z, Wang Y, Huang X, Ding S, Chen K, Wang N, et al. Formation and growth mechanism of ceftezole sodium monohydrate ring-banded spherulites. Cryst Growth Des. 2021;21:5967–75.
Aitipamula S, Shan LP, Gupta KM. Polymorphism and distinct physicochemical properties of the phloretin–nicotinamide cocrystal. CrystEngComm. 2022;24:560–70.
Zhou Y, Wang J, Xiao Y, Wang T, Huang X. The effects of polymorphism on physicochemical properties and pharmacodynamics of solid drugs. CPD. 2018;24:2375–82.
Shi Q, Moinuddin SM, Wang Y, Ahsan F, Li F. Physical stability and dissolution behaviors of amorphous pharmaceutical solids: role of surface and interface effects. Int J Pharm. 2022;625: 122098.
Censi R, Di Martino P. Polymorph impact on the bioavailability and stability of poorly soluble drugs. Molecules. 2015;20:18759–76.
Lee AY, Erdemir D, Myerson AS. Crystal polymorphism in chemical process development. Annu Rev Chem Biomol Eng. 2011;2:259–80.
Guo M, Sun X, Zhang S, Cai T. Modulation of solid-state chemical stability of gabapentin by pyridinecarboxylic acid. Pharm Res. 2022;39:2305–14.
Chemburkar SR, Bauer J, Deming K, Spiwek H, Patel K, Morris J, et al. Dealing with the impact of ritonavir polymorphs on the late stages of bulk drug process development. Org Process Res Dev. 2000;4:413–7.
Bučar D-K, Lancaster RW, Bernstein J. Disappearing polymorphs revisited. Angew Chem Int Ed. 2015;54:6972–93.
Rascol O, Perez-Lloret S. Rotigotine transdermal delivery for the treatment of Parkinson’s disease. Expert Opin Pharmacother. 2009;10:677–91.
Li D, Wang Y, Zong S, Wang N, Li X, Dong Y, et al. Unveiling the self-association and desolvation in crystal nucleation. IUCrJ. 2021;8:468–79.
Zong S, Wang J, Huang X, Wu H, Liu Q, Hao H. Formation and stabilization mechanism of mesoscale clusters in solution. IUCrJ. 2022;9:215–22.
Liu Y, Wang Y, Huang X, Li X, Zong S, Wang N, et al. Conformational selectivity and evolution affected by the desolvation process. Cryst Growth Des. 2022;22:1283–91.
Cedeno R, Cruz-Cabeza A, Drummond-Brydson R, K. Dudek M, Edkins K, Fichthorn K, et al. Controlling polymorphism: general discussion. Faraday Discuss. 2022;235:508–35.
Chakraborty J, Subash M, Thorat BN. Drying induced polymorphic transformation of pharmaceutical ingredients: a critical review of recent progresses and challenges. Drying Technol. 2022;40:2817–35.
Yadav K, Sachan AKr, Kumar S, Dubey A. Techniques for increasing solubility: a review of conventional and new strategies. Asian J Pharm Res Dev. 2022;10:144–53.
Park H, Kim J-S, Hong S, Ha E-S, Nie H, Zhou QT, et al. Tableting process-induced solid-state polymorphic transition. J Pharm Investig. 2022;52:175–94.
Sykes JE, Blondeau JM. Pradofloxacin: a novel veterinary fluoroquinolone for treatment of bacterial infections in cats. Vet J. 2014;201:207–14.
Lapointe G, Skepper CK, Holder LM, Armstrong D, Bellamacina C, Blais J, et al. Discovery and optimization of DNA gyrase and topoisomerase IV inhibitors with potent activity against fluoroquinolone-resistant gram-positive bacteria. J Med Chem. 2021;64:6329–57.
Penn-Nicholson A, Georghiou SB, Ciobanu N, Kazi M, Bhalla M, David A, et al. Detection of isoniazid, fluoroquinolone, ethionamide, amikacin, kanamycin, and capreomycin resistance by the Xpert MTB/XDR assay: a cross-sectional multicentre diagnostic accuracy study. Lancet Infect Dis. 2022;22:242–9.
Jia Y, Zhao L. The antibacterial activity of fluoroquinolone derivatives: an update (2018–2021). Eur J Med Chem. 2021;224: 113741.
Mathur P, Sanyal D, Callahan DL, Conlan XA, Pfeffer FM. Treatment technologies to mitigate the harmful effects of recalcitrant fluoroquinolone antibiotics on the environ- ment and human health. Environ Pollut. 2021;291: 118233.
Osman M, Albarracin B, Altier C, Gröhn YT, Cazer C. Antimicrobial resistance trends among canine Escherichia coli isolated at a New York veterinary diagnostic laboratory between 2007 and 2020. Prev Vet Med. 2022;208: 105767.
Merino-Gutierrez V, Puig J, Feo-Bernabe L. Successful treatment of 3 dogs with fluoroquinolone-resistant escherichia coli associated granulomatous colitis. Top Companion Anim Med. 2022;47: 100621.
Thomas H, Werner H, Hubert R. Crystal modification A of 8-cyano-1-cyclopropyl-7-(1s,6s-2,8-diazabicylo-[4.3.0]nonan-8-yl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid. CN1328558A. 2001.
Thomas H, Werner H, Hubert R. Crystal modification B of 8-cyano-1-cyclopropyl-7-(1s,6s-2,8-diazabicylo-[4.3.0]nonan-8-yl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid. CN1328556A. 2001.
Rast H, Himmler T. Crystal modification C of 8-cyano-1-cyclopropyl-7-(1s,6s-2,8-diazabicylo-[4.3.0]nonan-8-yl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid. CN1342160A. 2002.
Himmler T, Rast H. Crystal modification D of 8-cyano-1-cyclopropyl-7-(1s,6s-2,8-diazabicylo-[4.3.0]nonan-8-yl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid. CN1341116A. 2002.
Rast H, Heep I, Grunenberg A, Hallenbach W, Benet-Buchholz J. New crystalline form of 8-cyano-1-cyclopropyl-7-(1s,6s-2,8-diazabicyclo-[4.3.0]nonan-8-yl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid. CN1938304A. 2007.
Himmler T, Rast H. Semi-hydrochloride of 8-cyano-1-cyclopropyl-7-(1s,6s-2,8-diazabicylo-[4.3.0]nonan-8-yl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid. CN1328557A. 2001.
Sheldrick GM. A short history of SHELX. Acta Cryst A. 2008;64:112–22.
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst. 2009;42:339–41.
Connor LE, Delori A, Hutchison IB, Nic Daeid N, Sutcliffe OB, Oswald IDH. The ecstasy and the agony; compression studies of 3,4-methylenedioxymethamphetamine (MDMA). Acta Crystallogr B Struct Sci Cryst Eng Mater. 2015;71:3–9.
Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J Appl Cryst. 2015;48:3–10.
Thomas SP, Spackman PR, Jayatilaka D, Spackman MA. Accurate lattice energies for molecular crystals from experimental crystal structures. J Chem Theory Comput. 2018;14:1614–23.
Martin AD, Hartlieb KJ, Sobolev AN, Raston CL. Hirshfeld surface analysis of substituted phenols. Cryst Growth Des. 2010;10:5302–6.
Spackman MA, McKinnon JJ. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm. 2002;4:378–92.
Liu W, Hou B, Huang X, Zong S, Zheng Z, Li S, et al. Influence of intermolecular interactions and crystal structure on desolvation mechanisms of solvates. CrystEngComm. 2021;23:3557–68.
A B. On the polymorphism of pharmaceuticals and other molecular crystals. I, Microchim Acta. 1979;72:259–71.
Acknowledgements
This research is financially supported by National Natural Science Foundation of China (21908159).
Author information
Authors and Affiliations
Contributions
Wenlei Li: conceptualization, writing-review & editing. Lina Zhou: software. Beiqian Tian: conceptualization, software. Kui Chen: software. Yaoguang Feng: investigation. Ting Wang: supervision. Na Wang: supervision. Xin Huang: writing-review & editing. Hongxun Hao: resources, writing-review & editing.
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, W., Zhou, L., Tian, B. et al. Polymorphism of Pradofloxacin: Crystal Structure Analysis, Stability Study, and Phase Transformation Behavior. Pharm Res 40, 999–1012 (2023). https://doi.org/10.1007/s11095-023-03509-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03509-w